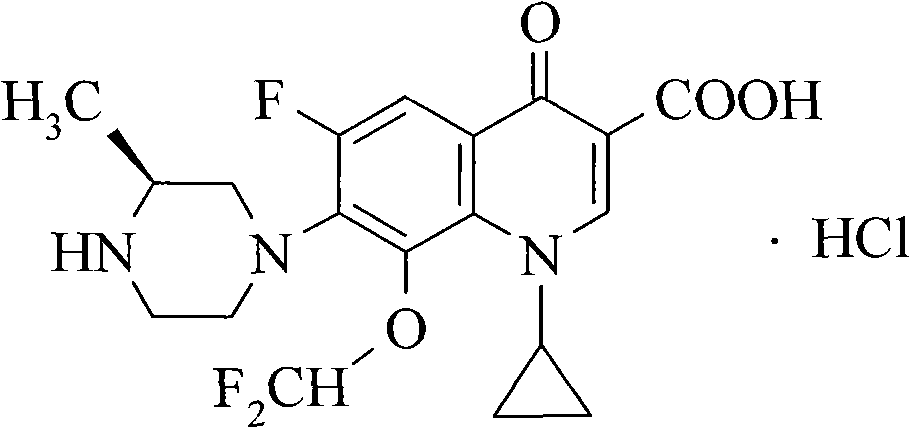
Cadrofloxacin hydrochloride-I crystal and preparation method thereof
A technology of caltrafloxacin and hydrochloric acid calfloxacin, which is applied to the I-type crystal of caltrafloxacin hydrochloride and its preparation field, can solve the problems of unfavorable crystal structure, easy agglomeration, fine crystallization of products, etc. Good storage, good chemical stability and controllable product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
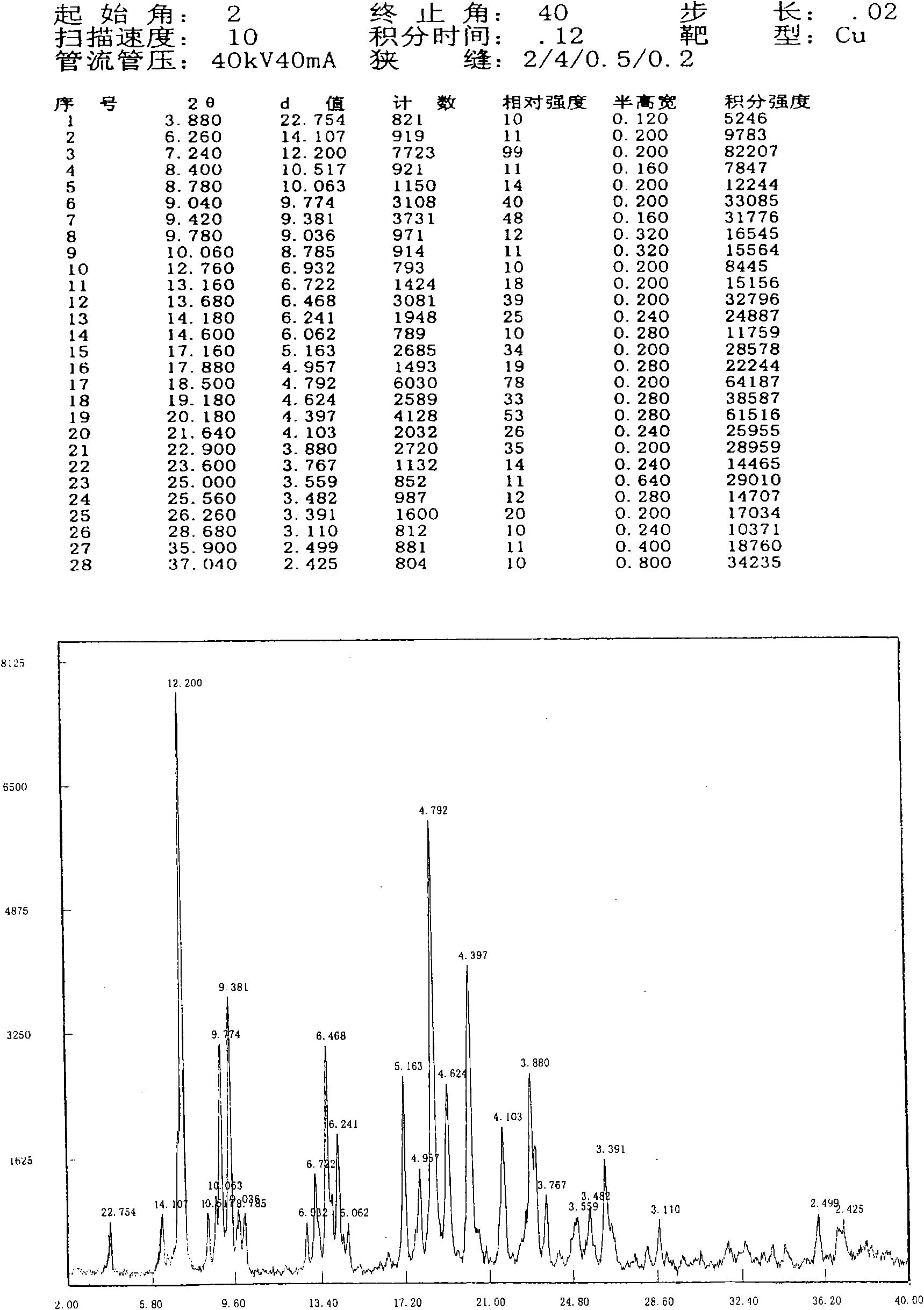
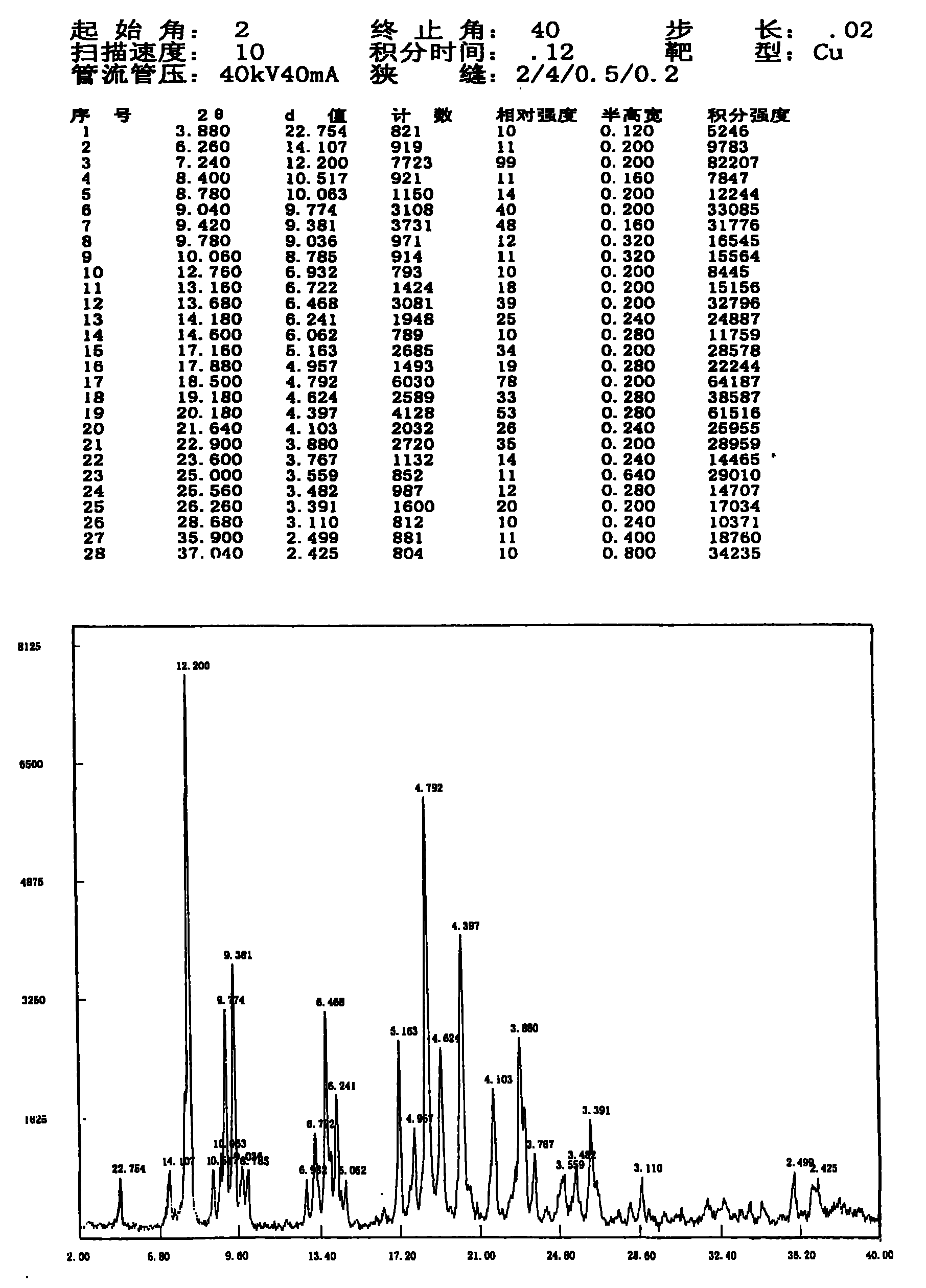
[0024] Add 2.00 g of cadrofloxacin hydrochloride into water (50 ml), heat to reflux to dissolve, filter and slowly cool to crystallize, and collect the precipitated crystals by filtration. The obtained crystals were dried at 40° C. under reduced pressure for 3 hours to obtain 1.17 g of crystals with a yield of 58.5%. The resulting sample contained 0.53% moisture; the X-ray diffraction spectrum of the crystallized sample can be found in figure 1 , is a type I crystal. The crystallization at about 3.88 (22.75), 7.24 (12.20), 9.04 (9.77), 9.42 (9.38), 13.16 (6.72), 13.68 (6.47), 14.18 (6.24), 17.16 (5.16), 17.88 (4.96), 18.50 There are characteristic peaks at (4.79), 19.18(4.62), 20.18(4.40), 21.64(4.10), 22.90(3.88), 26.26(3.39), 28.68(3.11) and 35.90(2.50). The stability investigation data of cadrofloxacin hydrochloride type I crystalline product are shown in Table 1.
Embodiment 2
[0026] Add 2.00 g of cadrofloxacin hydrochloride into water (50 ml), heat to reflux to dissolve, filter, concentrate under reduced pressure to about half the volume to crystallize, slowly cool to room temperature, and filter out the precipitated crystals. The obtained crystals were dried at 40° C. under reduced pressure for 3 hours to obtain 1.72 g of crystals with a yield of 86.0%. Gained sample contains 0.75% moisture; The X-ray diffraction pattern collection of samples of this crystallization and figure 1 Similar to type I crystal.
Embodiment 3
[0028] Add 10.0 g of cadrofloxacin hydrochloride into water (250 ml), heat to reflux to dissolve, filter, concentrate under reduced pressure to about half of the volume, rapidly rinse into absolute ethanol (800 ml), and collect the precipitated solid powder by filtration. The obtained solid was dried under reduced pressure at 40° C. for 5 hours to obtain 8.9 g of the product with a yield of 89.0%. The obtained sample contained 0.72% moisture; from the X-ray diffraction pattern of the crystalline sample, it can be seen that the product was an amorphous powder. The stability investigation data of cadrofloxacin hydrochloride amorphous product are shown in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com