Synthesis of class of amino acid type amphoteric water-soluble chiral phosphine ligand and application thereof in asymmetric catalytic hydrogenation
A chiral phosphine ligand, amino acid type technology, applied in asymmetric synthesis, organic compound/hydride/coordination complex catalyst, formation/introduction of carbonyl, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
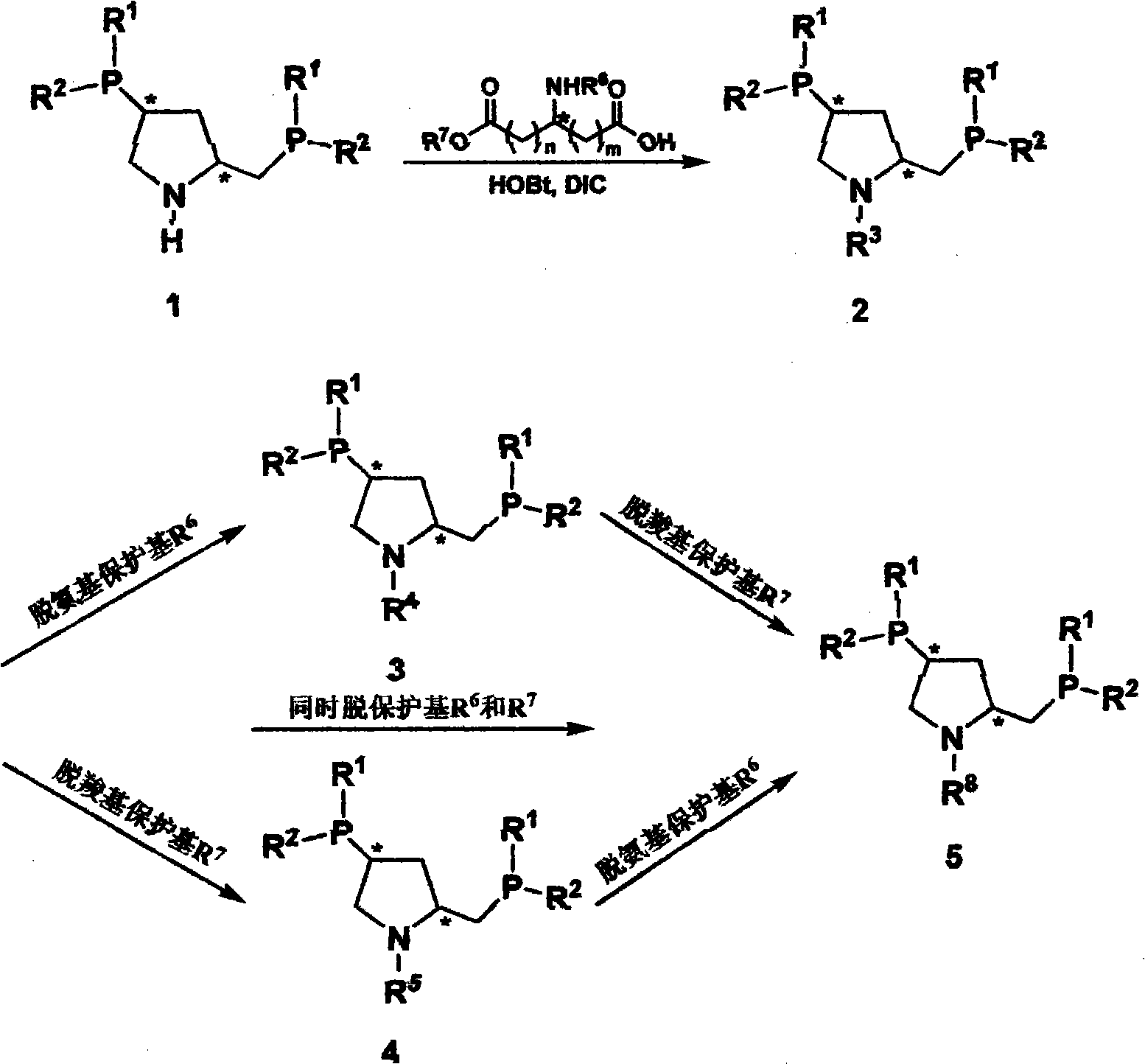
[0037] Synthesis of 2a
[0038] In a Schlenk flask equipped with a dropping funnel, reflux condenser and magnetic stirring, add 1.19 g (3.68 mmol) of N-Boc-L-aspartic acid-4-benzyl ester (Boc-Asp(OBz1)-OH) , 0.5g (3.68mmozl) 1-hydroxy-benzotriazole (HOBt) and 0.46g (3.68mmol) N,N-diisopropylcarbodiimide (DIC), then add 60mL of degassed CH 2 Cl 2 Stir to dissolve. Add 1.67 g (3.68 mmol) (2S, 4S)-2-diphenylphosphinomethyl-4-diphenylphosphinopyrrole (PPM) dropwise in 20 mL of CH 2 Cl 2 The solution was reacted at room temperature for 2 h under an argon atmosphere, and the solvent was dried under reduced pressure. Column chromatography (petroleum ether / ethyl acetate: 3:1) and vacuum drying gave 2.62 g of a white foamy solid with a yield of 93.6%.
[0039] [α] 20 D -25.0° (c 0.5, CH 2 Cl 2 ); 1 H NMR (CDCl 3 ): δ7.612~7.272(m, 25H), 5.354(d, J=8Hz, 1H), 5.036(s, 2H), 4.682(m, 1H), 4.103(m, 2H), 3.416(q, J =9.5Hz, 1H), 3.121(d, J=12Hz, 1H), 2.794~2.753(m, 2H), 2.557(dd, J...
Embodiment 2
[0049] Synthesis of 2b
[0050] In a Schlenk bottle equipped with a dropping funnel, reflux condenser and magnetic stirring, add 0.37 g (1.22 mmol) Boc-Glu(OtBu)-OH, 0.16 g (1.22 mmol) 1-hydroxyl-benzotriazole ( HOBt) and 0.15 g (1.22 mmol) N, N-diisopropylcarbodiimide (DIC), then add 30 mL of degassed CH 2 Cl 2 Stir to dissolve. Add 0.55 g (1.22 mmol) (2S,4S)-2-diphenylphosphinomethyl-4-diphenylphosphinopyrrole (PPM) dropwise in 15 mL of CH 2 Cl 2 The solution was reacted at room temperature for 2 h under an argon atmosphere, and the solvent was dried under reduced pressure. Column chromatography (petroleum ether / ethyl acetate: 3:1) and vacuum drying gave 0.83 g of white crystals, with a yield of 92.7%.
[0051] mp.175~176℃; 1 H NMR (CDCl 3 ): δ7.617~7.261(m, 20H), 5.379(d, 1H), 4.366(m, 1H), 4.155(m, 1H), 3.961(t, 1H), 3.456(m, 1H), 3.162( m, 1H), 2.826(m,), 2.296~2.204(m, 3H), 1.959~1.916(m, 2H), 1.854~1.792(m, 1H), 1.655~1.580(m, 1H), 1.407(s ,18H); 31 P NMR (CDCl...
Embodiment 3
[0056] Synthesis of 2c
[0057] In a Schlenk bottle equipped with a dropping funnel, reflux condenser and magnetic stirring, add 0.234 g (0.81 mmol) Boc-Asp-OtBu, 0.11 g (0.81 mmol) 1-hydroxy-benzotriazole (HOBt) and 0.10 g (0.81 mmol) N,N'-diisopropylcarbodiimide (DIC), then add 30 mL of degassed CH 2 Cl2 Stir to dissolve. Add 0.37 g (0.81 mmol) (2S, 4S)-2-diphenylphosphinomethyl-4-diphenylphosphinopyrrole (PPM) dropwise in 10 mL of CH 2 Cl 2 The solution was reacted at room temperature for 2 h under an argon atmosphere, and the solvent was dried under reduced pressure. Column chromatography (petroleum ether / ethyl acetate: 3:1) and vacuum drying gave 0.55 g of white crystals, with a yield of 94.0%.
[0058] mp.189~191℃; 1 H NMR (CDCl 3 ): δ7.610~7.290(m, 20H), 5.715(d, 1H), 4.330(m, 1H), 4.268~4.142(m, 1H), 3.517(t, 1H), 3.296(m, 1H), 3.090(m, 1H), 2.878~2.787(m, 2H), 2.280~2.213(m, 3H), 1.924(m, 1H), 1.440(s, 18H); 31 P NMR (CDCl 3 ): δ-6.150, -9.142, -22.246, -22.75...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com