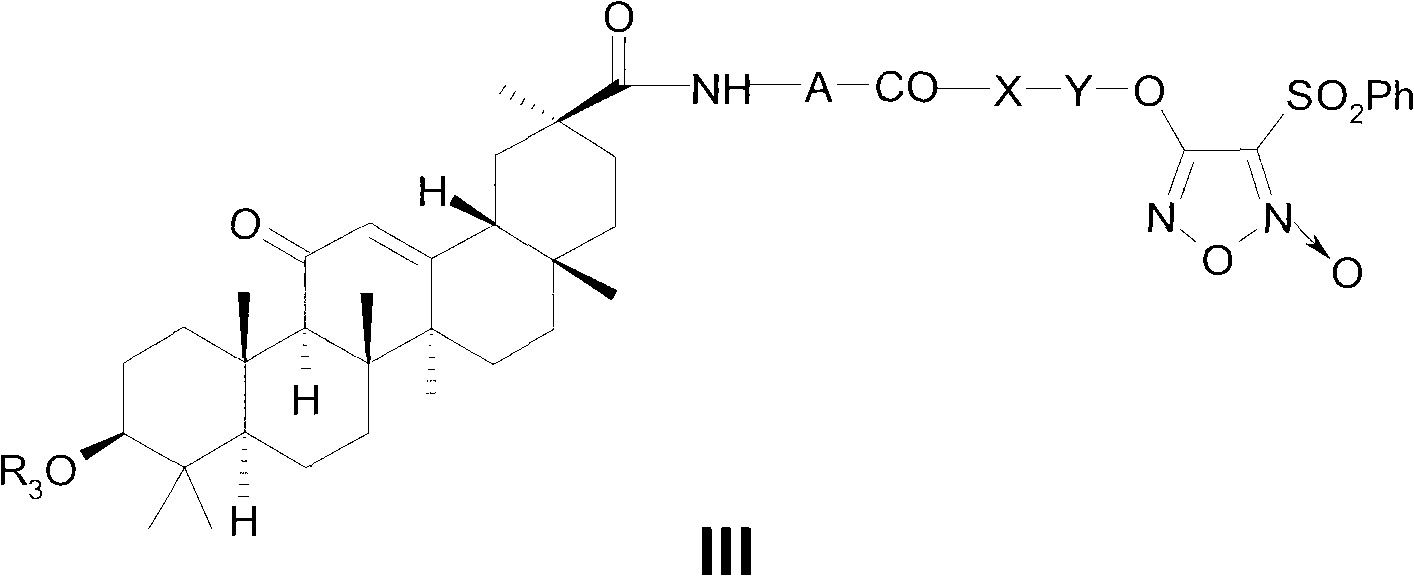
Novel glycyrrhetinic acid derivative, and preparation method and medicinal uses thereof
A technology of glycyrrhetinic acid and derivatives, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
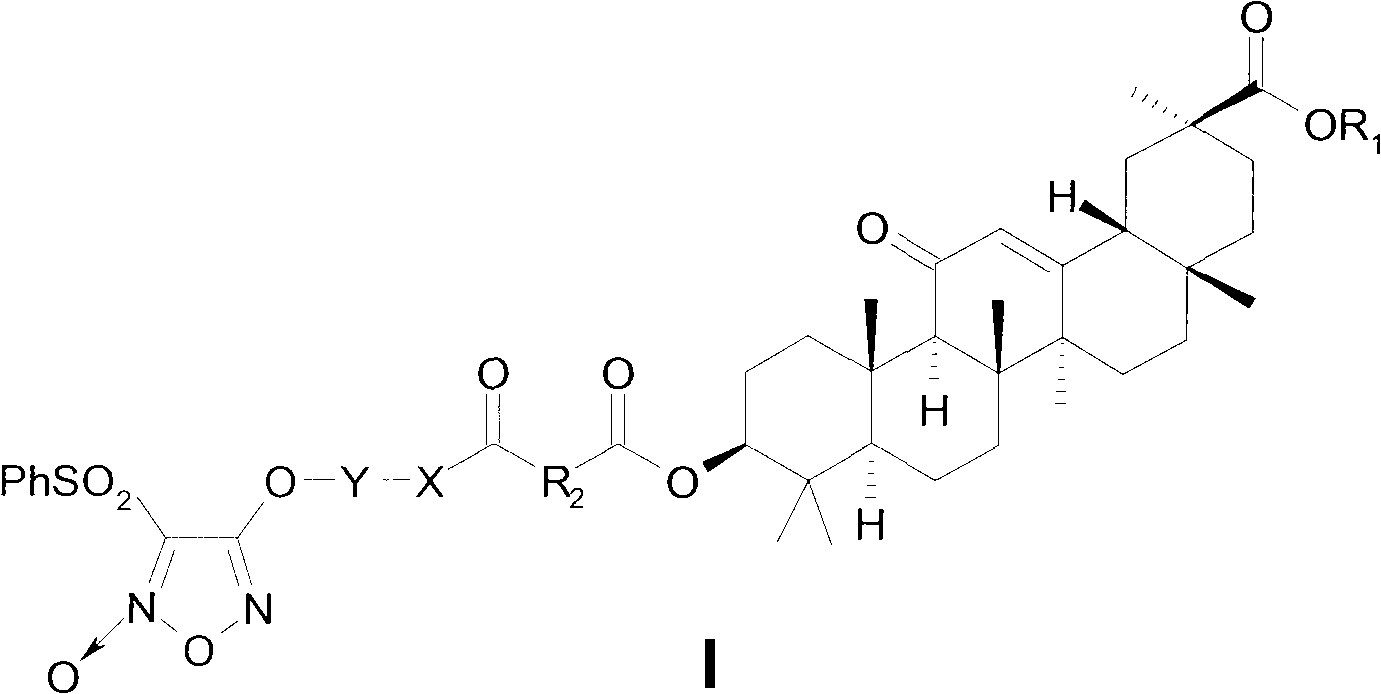
[0131] Preparation of methyl 3-O-succinic acid monoacylglycyrrhetinate (1)
[0132] Add 0.48g (1.00mmol) methyl glycyrrhetinate, 0.60g (6.00mmol) succinic anhydride, 0.16g (1.30mmol) DMAP into 20mL anhydrous CH 2 Cl 2 refluxed for 15 hours, the reaction solution was washed three times with water, concentrated, and recrystallized from methanol / water to obtain 0.57 g of white powder (1), yield 98%, mp: 260-262°C.
[0133] Preparation of 2-[(4-benzenesulfonyl-5-oxo-1,2,5-oxadiazole-3-)oxy]ethanol (2a)
[0134] Dissolve 3 mL (50 mmol) of ethylene glycol and 1.85 g (5 mmol) of 2-oxo-3,4-diphenylsulfonyl-1,2,5-oxadiazole in 20 mL of THF, cool in an ice bath, and drop in 2.5 mol / L NaOH Solution 2mL, react at room temperature for 0.5h, add 1mL of 2.5mol / L NaOH solution, continue to stir until the reaction of the raw materials is complete, pour 80mL of water, extract with ethyl acetate (3×20mL), wash with saturated brine, and dry over anhydrous sodium sulfate. Concentrate and recrys...
Embodiment 3
[0143] Preparation of 1-methyl-3-[(4-benzenesulfonyl-5-oxo-1,2,5-oxadiazole-3-)oxy]propanol (2c)
[0144] Referring to the preparation method of 2a, a white solid was prepared from 1,3-butanediol with a yield of 88%, mp: 101-103°C.
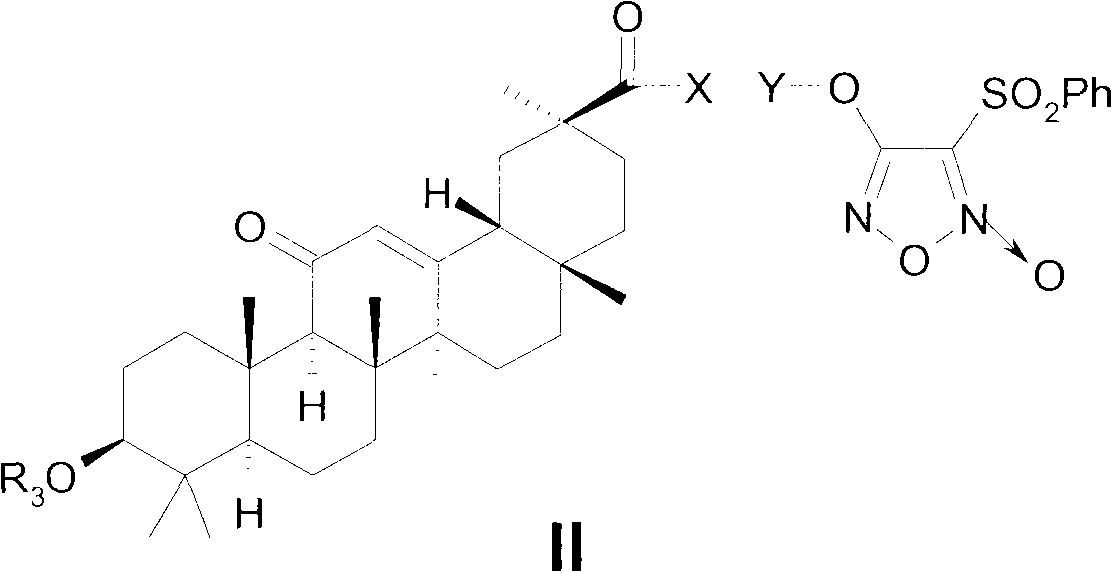
[0145] 3-{4-[1-methyl-3-[(4-benzenesulfonyl-5-oxo-1,2,5-oxadiazole-3-)oxy]propoxy]-1,4-di Oxobutoxy}glycyrrhetinic acid methyl ester (I 3 ) preparation
[0146] Refer to I 1 The preparation method is prepared by reacting 3-O-succinic acid monoacylglycyrrhetinic acid methyl ester (1) with 2c, white solid, yield 65%, mp: 66~68°C. ESI-MS(m / z): 881.5[M+H] + ; IR(KBr, cm -1 )ν: 2950, 2871, 1730, 1658, 1616, 1552, 1454, 1380, 1164; 1H-NMR (300MHz, CDCl 3 )δ: 0.80~1.56(m, 21H, 7CH 3 ), 2.35(s, 1H, C 9 -H), 2.68(m, 4H, CO(CH 2 ) 2 ), 3.69 (s, 3H, OCH 3 ), 4.50~4.53 (m, 3H, C 3 -H,OCH 2 ), 4.61~4.64 (m, 1H, OCH), 5.66 (s, 1H, C 12 -H), 7.60~7.65(m, 2H, ArH), 7.76(m, 1H, ArH), 8.06~8.08(m, 2H, ArH).
Embodiment 4
[0148] Preparation of 4-[(4-Benzenesulfonyl-5-oxo-1,2,5-oxadiazol-3-)oxy]butanol (2d)
[0149] Referring to the preparation method of 2a, a white solid was obtained from 1,4-butanediol with a yield of 89%, mp: 70-72°C.
[0150] 3-{4-[4-[(4-Benzenesulfonyl-5-oxo-1,2,5-oxadiazole-3-)oxy]butoxy]-1,4-dioxobutoxy }Methyl glycyrrhetinate (I 4 ) preparation
[0151] Refer to I 1 The preparation method is prepared by reacting 3-O-succinic acid monoacylglycyrrhetinic acid methyl ester (1) with 2d, white solid, yield 68%, mp: 78~80℃. ESI-MS(m / z): 881.3[M+H] + ; IR(KBr, cm -1 )ν: 2954, 2869, 1731, 1660, 1616, 1552, 1452, 1373, 1163; 1 H-NMR (300MHz, CDCl 3 )δ: 0.80~1.45(m, 21H, 7CH 3 ), 2.35(s, 1H, C 9 -H), 2.64(m, 4H, CO(CH 2 ) 2 ), 3.69 (s, 3H, OCH 3 ), 4.17~4.21(t, 2H, OCH 2 , J=6.0Hz), 4.43~4.47(t, 2H, OCH 2 , J=6.0Hz), 4.52(m, 1H, C 3 -H), 5.66(s, 1H, C 12 -H), 7.62~7.65(m, 2H, ArH), 7.76(m, 1H, ArH), 8.05~8.07(m, 2H, ArH).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Mp | aaaaa | aaaaa |
| Mp | aaaaa | aaaaa |
| Mp | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com