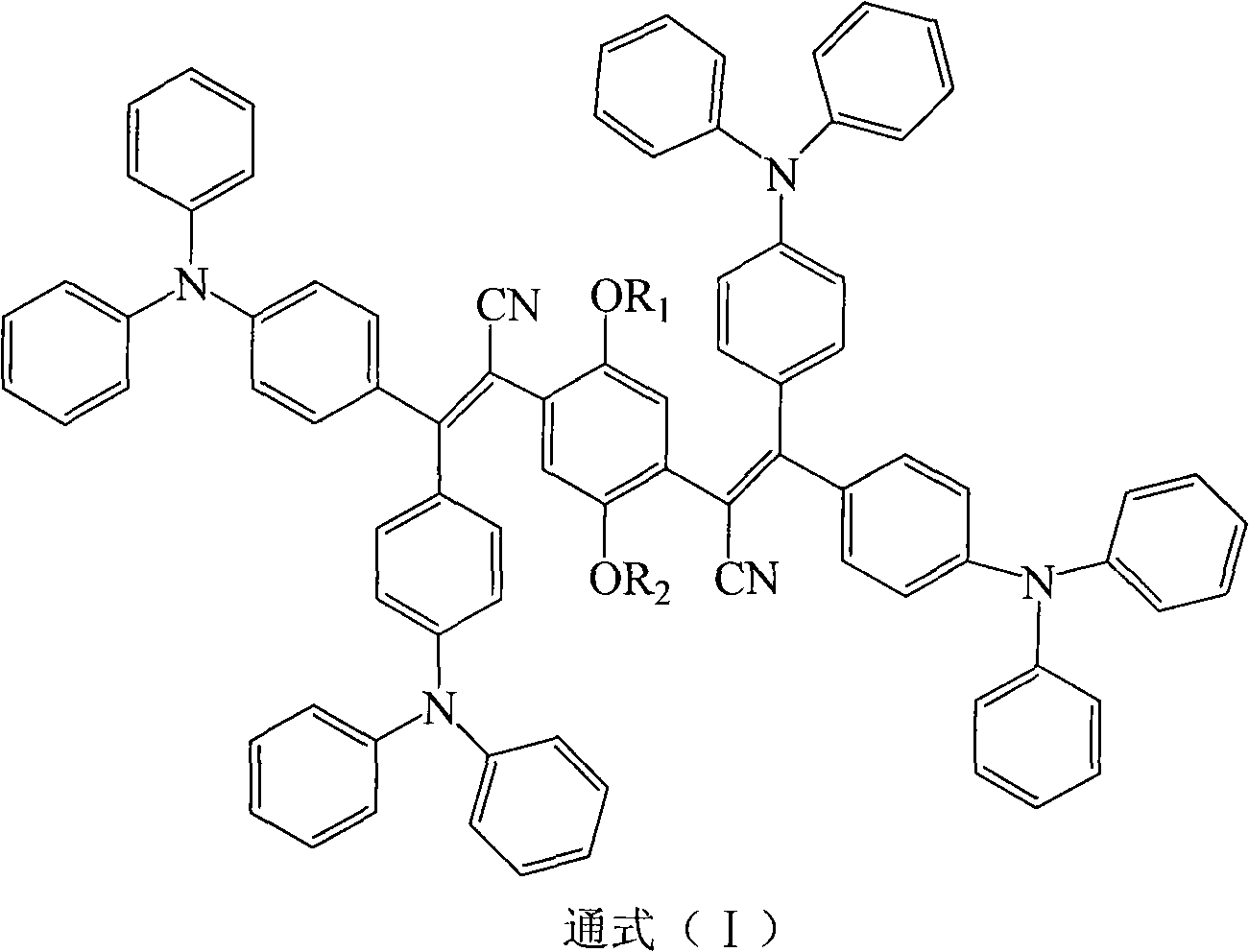
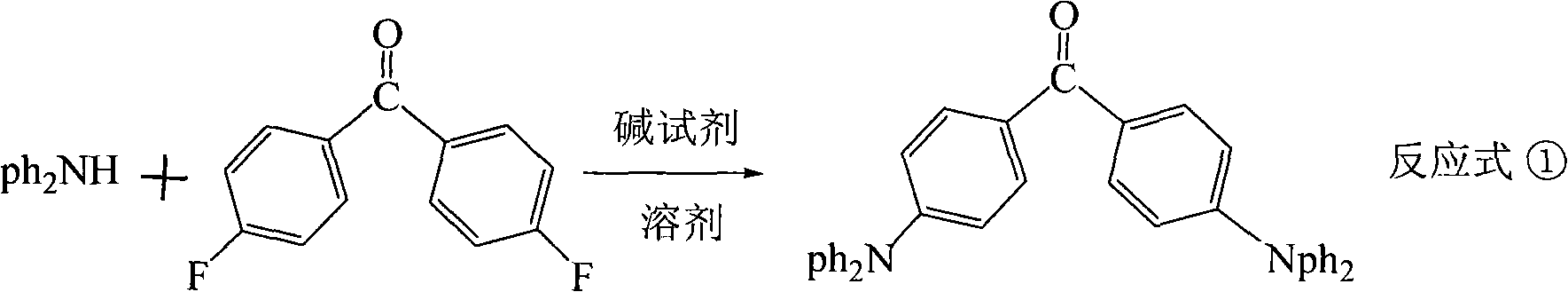
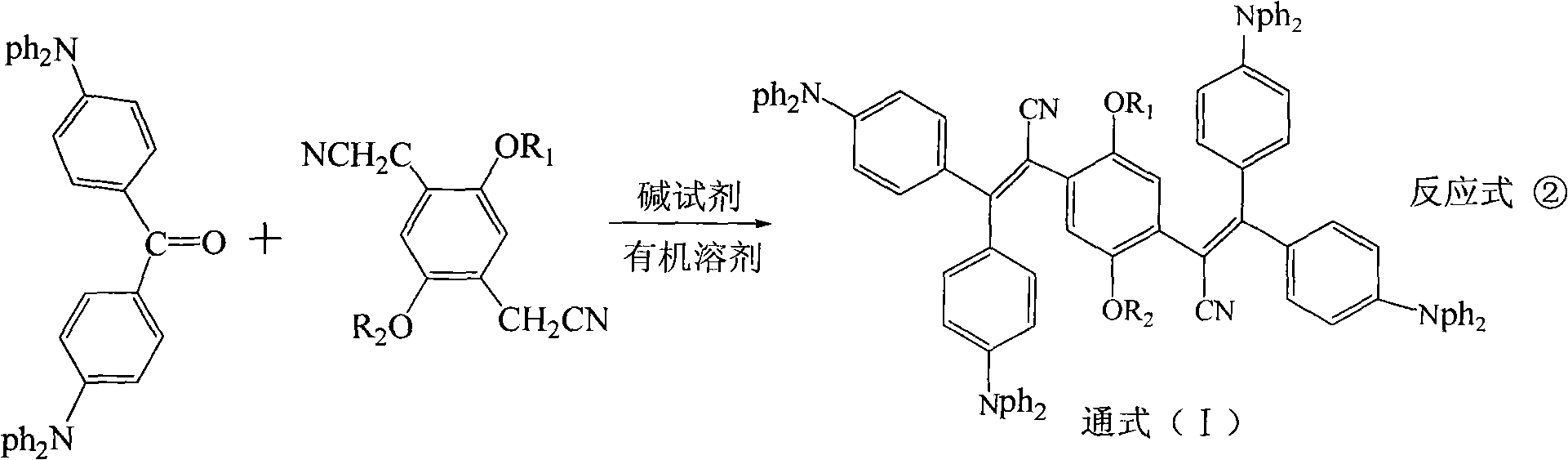
Triphenylethylene compound containing cyanogen and preparation method thereof
A technology of triphenylene and compound, applied in the field of cyano group-containing triphenylene compound and preparation thereof, can solve the problems of weak photoluminescence, decreased luminous efficiency, increased luminous intensity and the like, achieves wide sources and easy control of synthesis conditions Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Embodiment 1 The preparation of cyanotriphenylethylene compound I-1
[0029]
[0030] Step one 4, the synthesis of 4'-two (diphenylamino) benzophenone intermediates: in a four-necked reaction flask equipped with a stirrer, add 30 milliliters of hexamethylphosphoramide, 8.5 grams (0.05 moles) of diphenhydramine Aniline and 0.052 moles of sodium tert-butoxide, stirred to disperse evenly, N 2 Under protection, heat up at 60-80°C and keep it warm for 2 hours to remove tert-butanol. Thereafter, 4.4 g (0.02 mol) of 4,4'-difluorobenzophenone was added in batches, and the reaction was completed at 100-130° C. for 6 hours. Cool the reaction product system to room temperature, add ethanol, stand for crystallization, filter, use ethanol / acetone (volume ratio V / V=3 / 7) mixed solvent to recrystallize the filter cake, and vacuum dry to obtain 11.75 grams of yellow 4,4 '-Bis(diphenylamino)benzophenone intermediate, the yield is 91.7%, and the melting point is 173-175°C. Elemental...
Embodiment 2
[0032] The preparation of embodiment 2 cyano-triphenylethylene compounds I-2
[0033]
[0034] According to the preparation method and operating procedure of Example 1, the raw material 2,5-dimethoxy-1,4-benzenediacetonitrile in Step 2 in Example 1 was replaced with 2-methoxy-5-n-butoxy- 1,4-benzenediacetonitrile, the bright green cyano-triphenylethylene compound I-2 of the present invention can be obtained. Tg121°C was measured by TGA method, and Td450°C was measured by DSC method. Its concentration is 4.5×10 -5 The mol / L xylene solution has an ultraviolet-visible spectrum characteristic peak λmax=402nm, and a fluorescence spectrum characteristic peak λmax=494nm with an excitation wavelength of 400nm.
Embodiment 3
[0035] The preparation of embodiment 3 cyano-triphenylethylene compounds I-3
[0036]
[0037] According to the preparation method and operation procedure of Example 1, the raw material 2,5-dimethoxy-1,4-benzenediacetonitrile in Step 2 of Example 1 was replaced with 2,5-di-neopentyloxy-1,4- Phenyldiacetonitrile, the bright green cyanotriphenylethylene compound I-3 of the present invention can be obtained. Tg102°C was measured by TGA method, and Td435°C was measured by DSC method. Its concentration is 4.5×10 -5 The mol / L xylene solution has an ultraviolet-visible spectrum characteristic peak λmax=401nm, and a fluorescence spectrum characteristic peak λmax=496nm with an excitation wavelength of 400nm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com