Method for simultaneously carrying out chiral separation analysis on anisodamine, atenolol and metoprolol
A technology for anisodamine and chiral separation, which is applied in the field of chiral separation and analysis of chiral drugs, and can solve the problem of separation and analysis of metoprolol enantiomers and atenolol enantiomers and other problems, to achieve the effect of low detection cost, high separation efficiency and less use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Prepare NaH as follows 2 PO 4 -Na 2 HPO 4 Buffer solution:
[0043] Prepare NaH with a concentration of 200mmol / L respectively 2 PO 4 solution and 200mmol / L Na 2 HPO 4 Solution, after mixing the two solutions, dilute to a concentration of 100mmol / L with secondary water, then adjust the pH of the mixed solution to 7.0 for later use.
Embodiment 2
[0045] Follow the steps below to prepare HAc-NaAc buffer solution:
[0046] First prepare a NaAc solution with a concentration of 200mmol / L, then dilute it to 57.6mmol / L with secondary water, and then adjust the pH value of the solution to 5.3 with glacial acetic acid for later use.
Embodiment 3
[0048] The instruments used are: an uncoated fused silica capillary column with an inner diameter of 50 μm, a Pt disk working electrode with a diameter of 500 μm, an Ag / AgCl reference electrode, a Pt wire counter electrode, and MPI- A type capillary electrophoresis electrochemiluminescence detector.
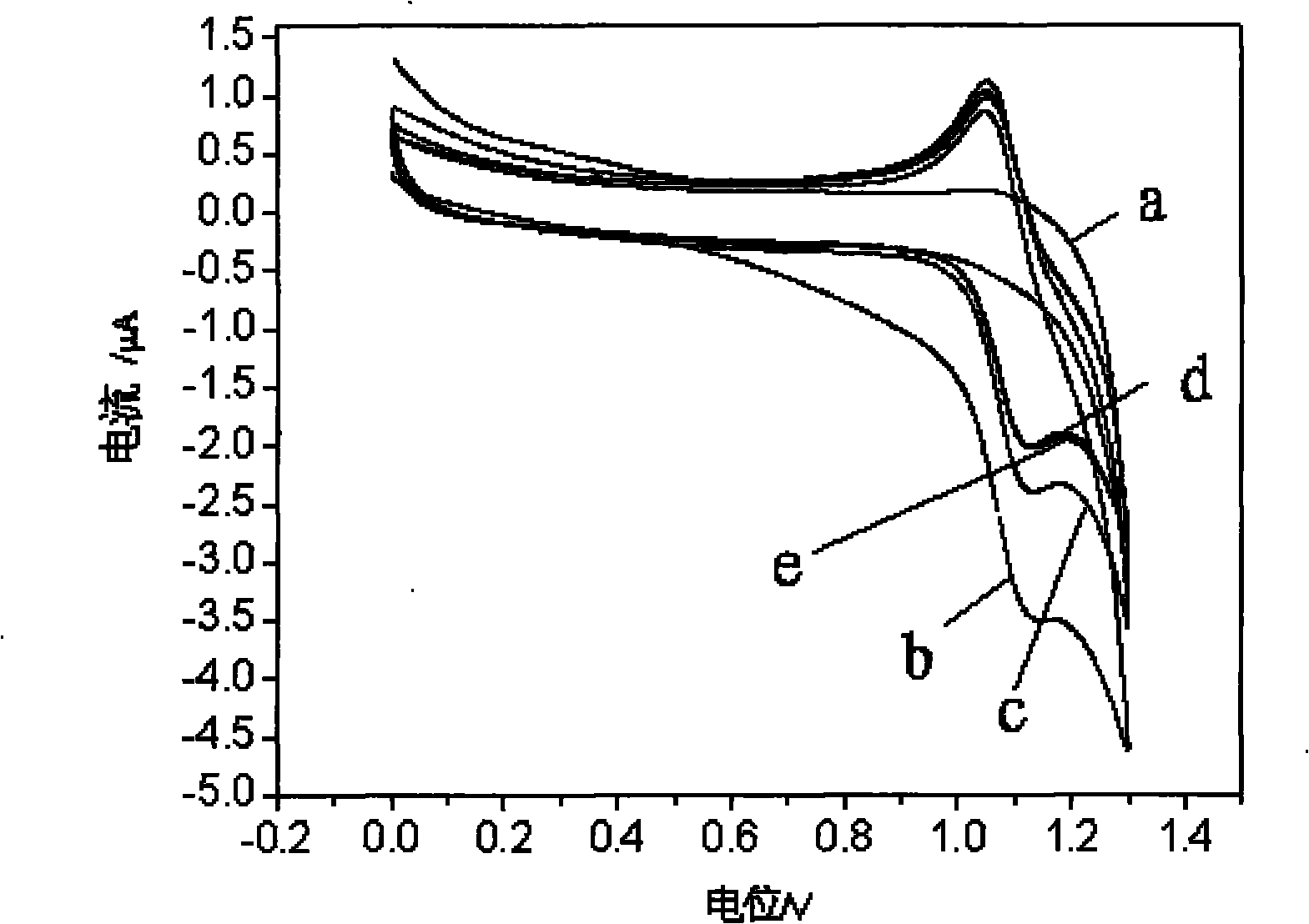
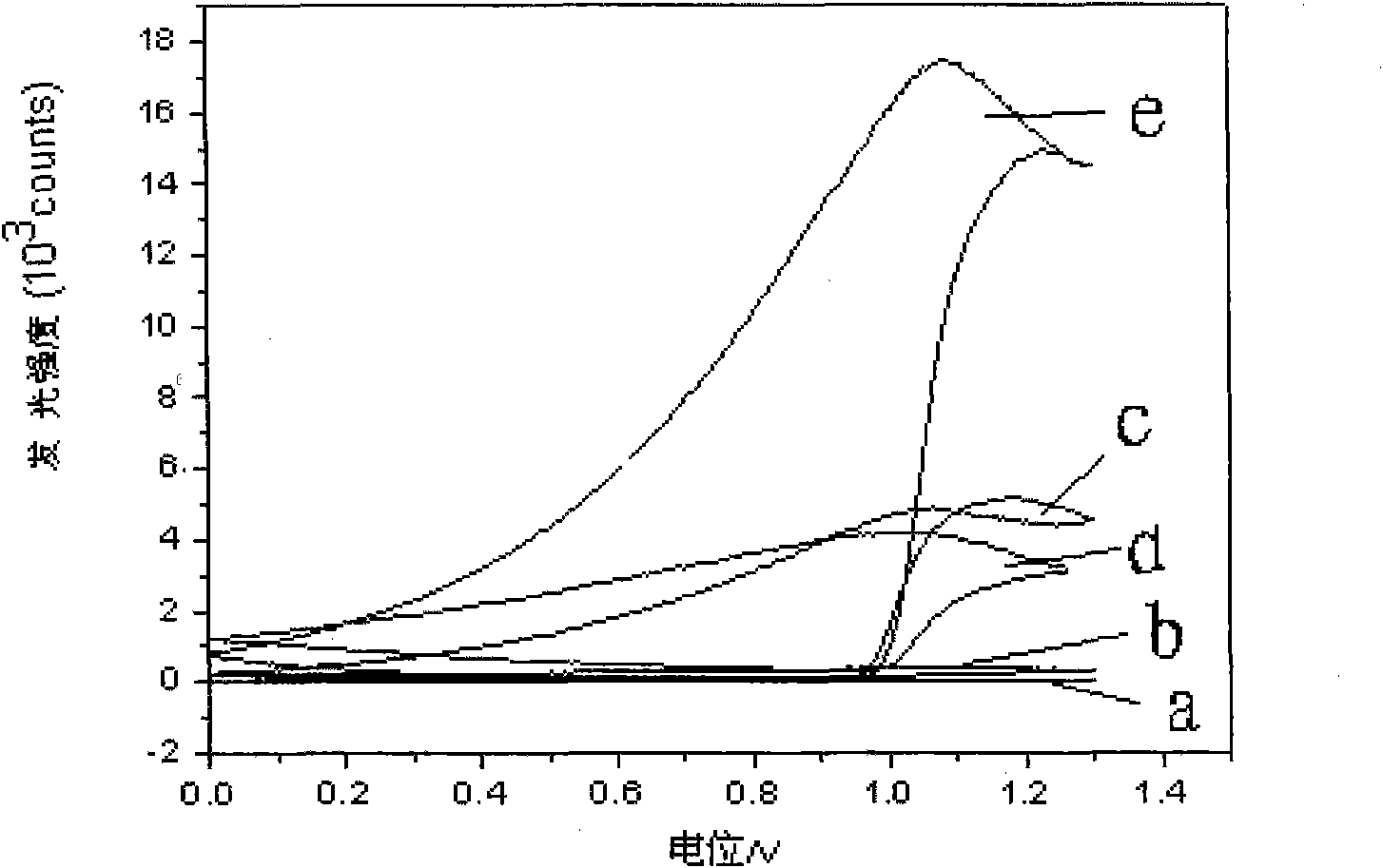
[0049] With the NaH of embodiment 1 preparation 2 PO 4 -Na 2 HPO 4 The buffer solution is the background electrolyte, the scanning potential is 0-1.30V, and the cyclic voltammetry and electrochemiluminescence curves are recorded at steady state, see figure 1 a curve in and figure 2 a curve in
[0050] Add 5mmol / L ruthenium terpyridine to the background electrolyte, the scanning potential is 0-1.30V, record the cyclic voltammetry curve and the electrochemiluminescence curve when stable, see figure 1 The b curve in and figure 2 The b curve in;
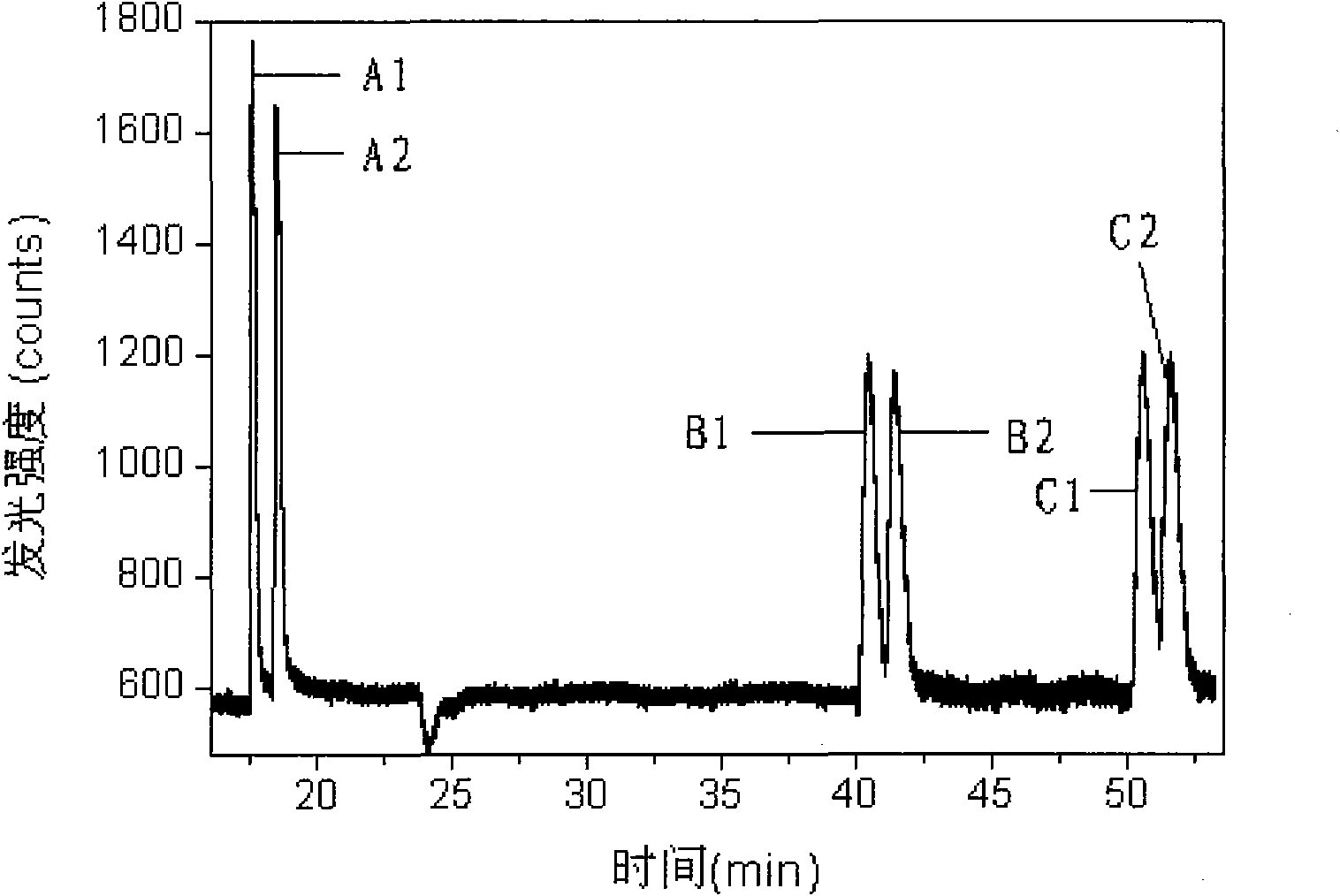
[0051] Add 0.3mmol / L anisodamine, 0.3mmol / L atenolol and 0.3mmol / L metoprolol to the solution containing ruthenium terpyridyl, t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
| The inside diameter of | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com