Oral solid preparation containing clopidogrel hydrogen sulfate
A technology of clopidogrel hydrogen sulfate and solid preparation, applied in the field of medicine, can solve problems such as increase in substance, decrease in stability, decrease in tablet dissolution rate, etc. Effective therapeutic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
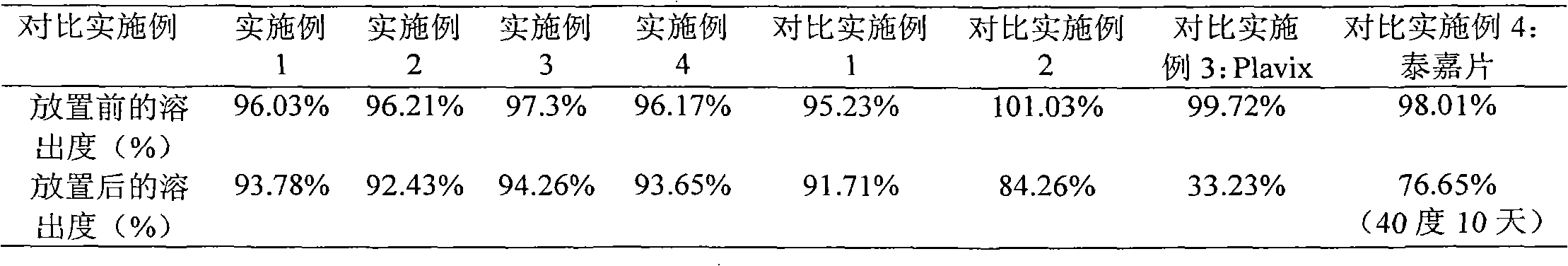
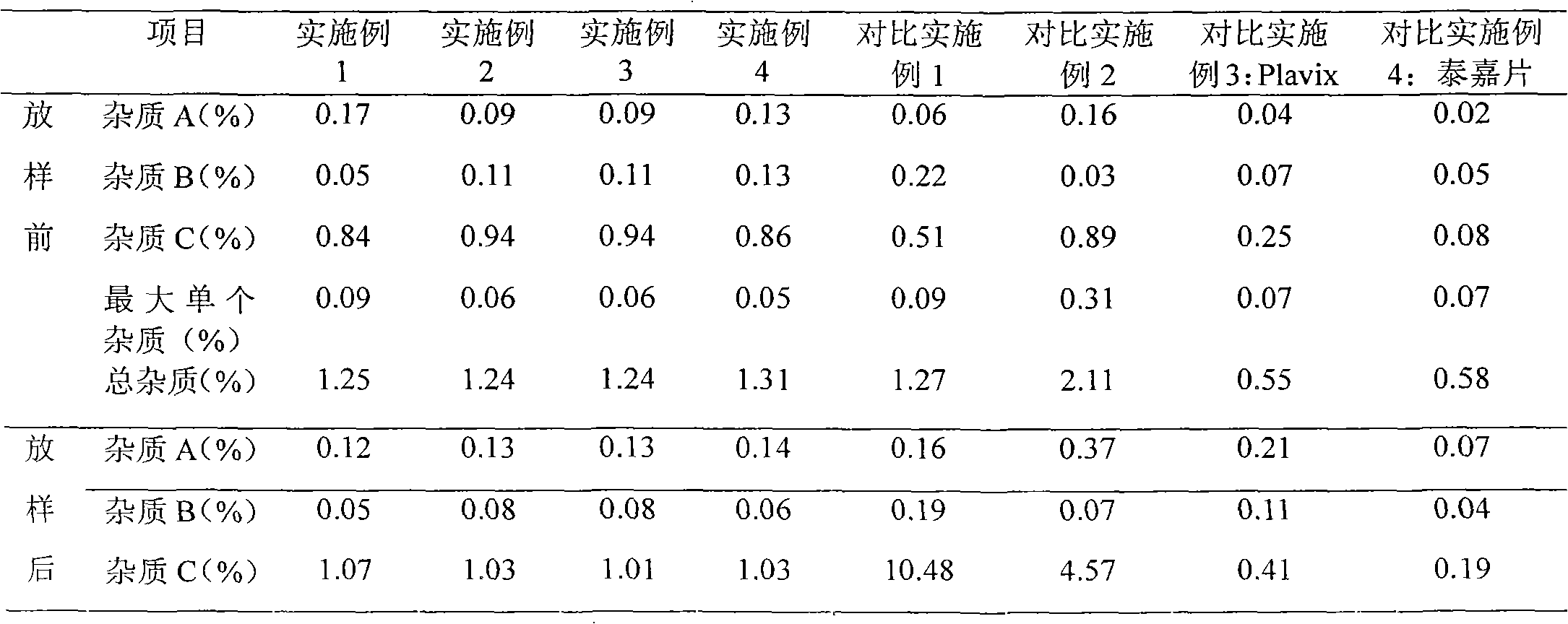
Embodiment 1
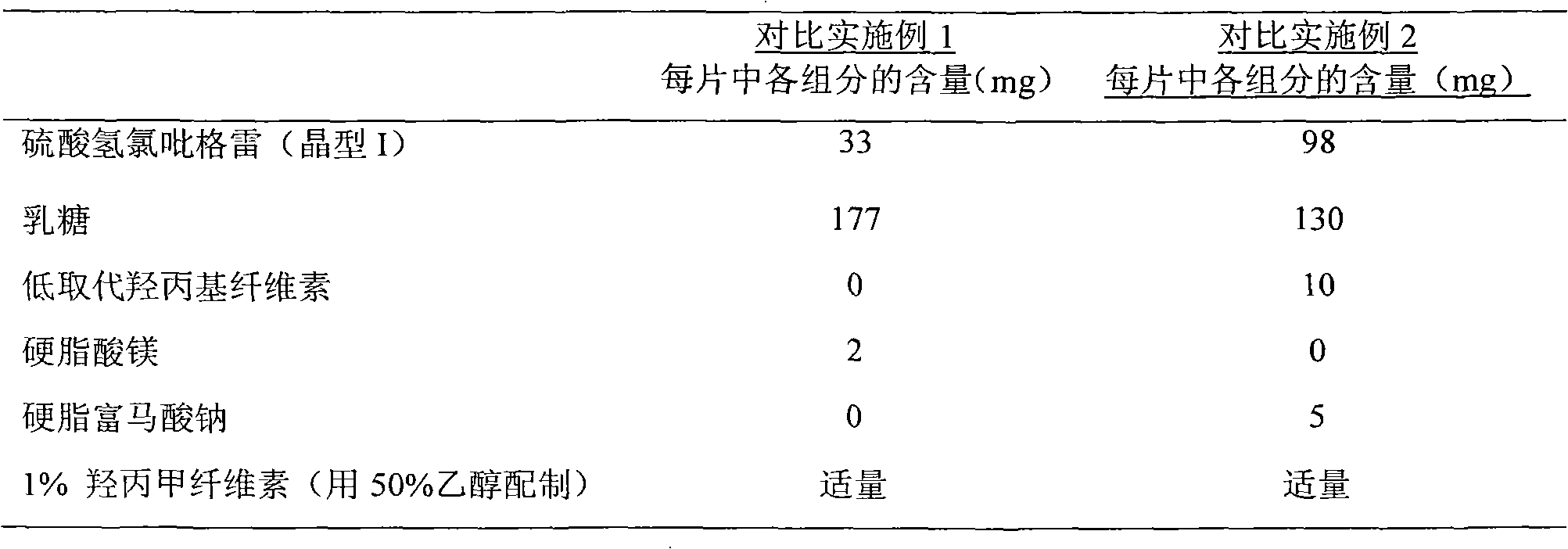
[0071] Clopidogrel bisulfate tablet 1 recipe
[0072] Preparation Content of each component in each tablet (mg)
[0073] Clopidogrel Bisulfate 33 (equivalent to Clopidogrel 25mg)
[0074] Lactose 177
[0076] Light liquid paraffin 0.5
[0077] 1% hypromellose (prepared with 50% ethanol) appropriate amount
[0078] Preparation method: uniformly mix clopidogrel hydrogen sulfate (crystalline form I) and lactose, add 1% hypromellose to prepare wet granules, and dry the granules at 50°C. Add the mixture of light liquid paraffin and talcum powder, mix well and press into tablets.
Embodiment 2
[0080] Clopidogrel Bisulfate Tablets 2 Formulations
[0081] Preparation Content of each component in each tablet (mg)
[0082] Clopidogrel Bisulfate 33 (equivalent to Clopidogrel 25mg)
[0083] Lactose 330
[0084] Low-substituted hydroxypropyl cellulose 12
[0085] Talc 32
[0086] Light liquid paraffin 0.8
[0087] Preparation method: Mix all materials evenly and directly compress into tablets.
Embodiment 3
[0089] Clopidogrel Bisulfate Tablets 3 Formulations
[0090] Preparation Content of each component in each tablet (mg)
[0091] Clopidogrel Bisulfate 98 (equivalent to Clopidogrel 75mg)
[0092] Lactose 130
[0093] Low-substituted hydroxypropyl cellulose 10
[0094] Talc 12
[0095] Light liquid paraffin 0.8
[0096] 1% hypromellose (prepared with 50% ethanol) appropriate amount
[0097] Preparation method: Mix clopidogrel hydrogen sulfate (form I) and lactose evenly, then add low-substituted hydroxypropyl cellulose and mix evenly; add 1% hypromellose solution, use wet granulation, and dry at 50 degrees Granules; add a mixture of light liquid paraffin and talcum powder, mix well and compress into tablets.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com