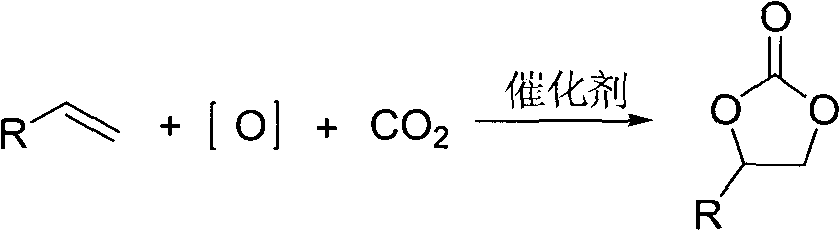Cyclic carbonate compound and synthesis method thereof
A cyclocarbonate and compound technology, applied in the field of cyclocarbonate and its preparation, can solve problems such as high reaction temperature and long reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
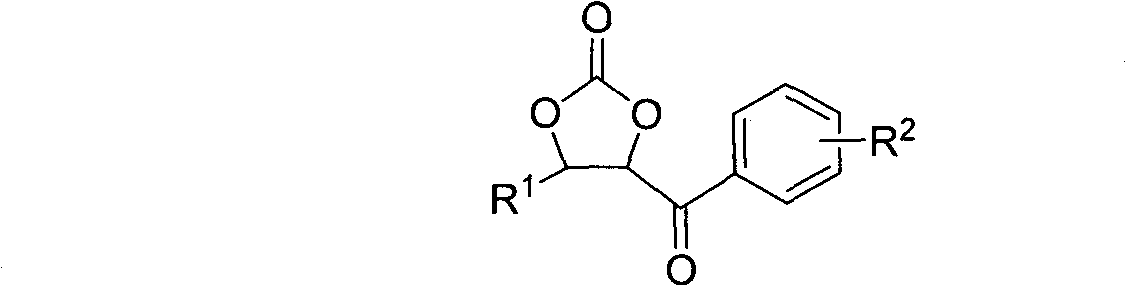
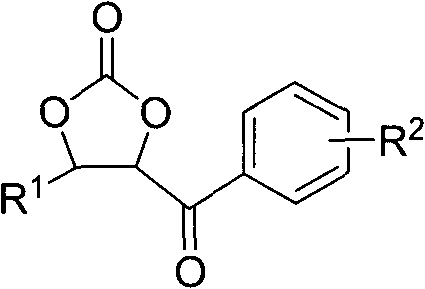
[0032] In a 25ml three-necked flask, benzaldehyde (2mmol, 0.212g) and α-bromoacetophenone (4mmol, 0.796g) were dissolved in anhydrous dioxane solution (5ml), stirred under a magnetic sub and 35 Under the water bath temperature of ℃, pass through the carbon dioxide gas flow under normal pressure (flow rate: 200-300ml / min), then LDA (2.5ml, 1M) is dripped into the above solution through the constant pressure funnel (the dripping process takes 2.5min ), and then the reaction system was reacted for ten minutes at a water bath temperature of 35° C. and a stable carbon dioxide gas flow condition. After the reaction was completed, the reaction was quenched by saturated ammonium chloride solution, extracted with ethyl acetate (10ml×3), dried over magnesium sulfate, and the solvent was evaporated under reduced pressure. The crude product was purified by silica gel (100-200 mesh) column chromatography to obtain the target product-cyclocarbonate-4-phenyl-5-benzoyl-1,3-dioxol-2-one. Chro...
Embodiment 2
[0036] In the same apparatus as used in Example 1, under the same conditions, except that benzaldehyde was changed to p-methoxybenzaldehyde (0.272 g). 0.137 g of cyclocarbonate-4-(4′-methoxyphenyl)-5-benzoyl-1,3-dioxolan-2-one was obtained, the yield was 23%, and the product had structural formula 9.
[0037]
[0038] Formula 9
Embodiment 3
[0040] In the same apparatus as used in Example 1, under the same conditions, except that benzaldehyde was changed to o-methoxybenzaldehyde (0.272 g). Obtain 0.482g of cyclocarbonate-4-(2'-methoxyphenyl)-5-benzoyl-1,3-dioxol-2-one, the yield is 81%, and the product structure is as follows: 10.
[0041]
[0042] Formula 10
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com