Porphyrin modified by diethylenetriamine pentaacetic acid gadolinium and preparation method and application thereof
A technology of diethylenetriaminepentaacetic acid and porphyrin, applied in the field of porphyrin, can solve the problem that the biocompatibility of porphyrin is not much improved, and achieve the effects of high enhancement effect, strong fluorescence effect and high relaxation effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
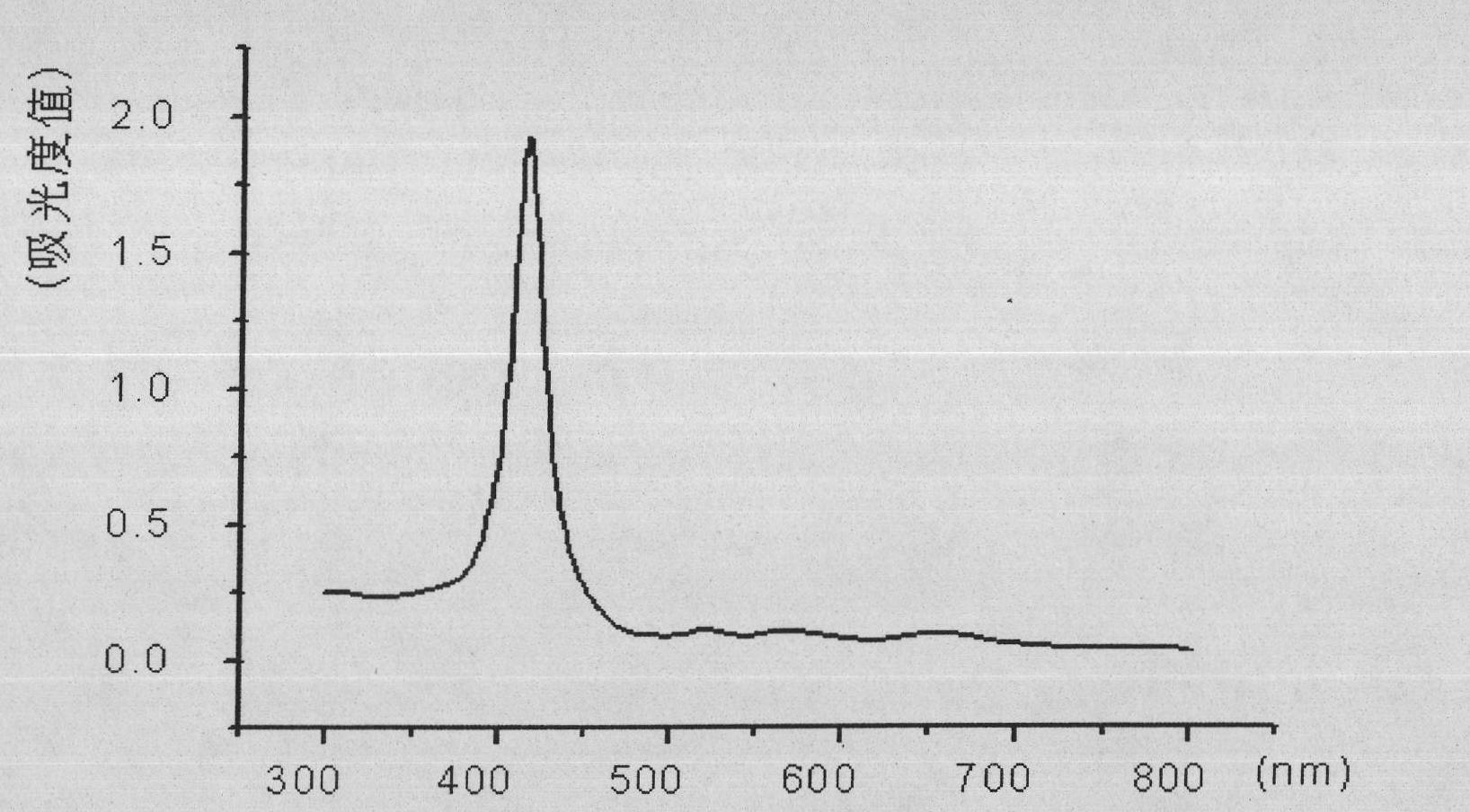
[0043] (1) 0.2g 5,10,15,20-tetra-[p-aminophenyl] porphyrin, 1.4g diethylenetriaminepentaacetic acid bicyclic anhydride, 1.0ml triethylamine dissolved in 15ml dimethyl In sulfoxide, under the catalysis of 4,4-dimethylaminopyridine, stir at room temperature for 12h. After the reaction is complete, add 30mL of water, let it stand, and precipitate the product. Suction filtration to obtain the crude product. Washing with dichloromethane for 3 times and ethanol for 3 times, drying and recrystallizing to obtain a porphyrin compound modified with diethylenetriaminepentaacetic acid;
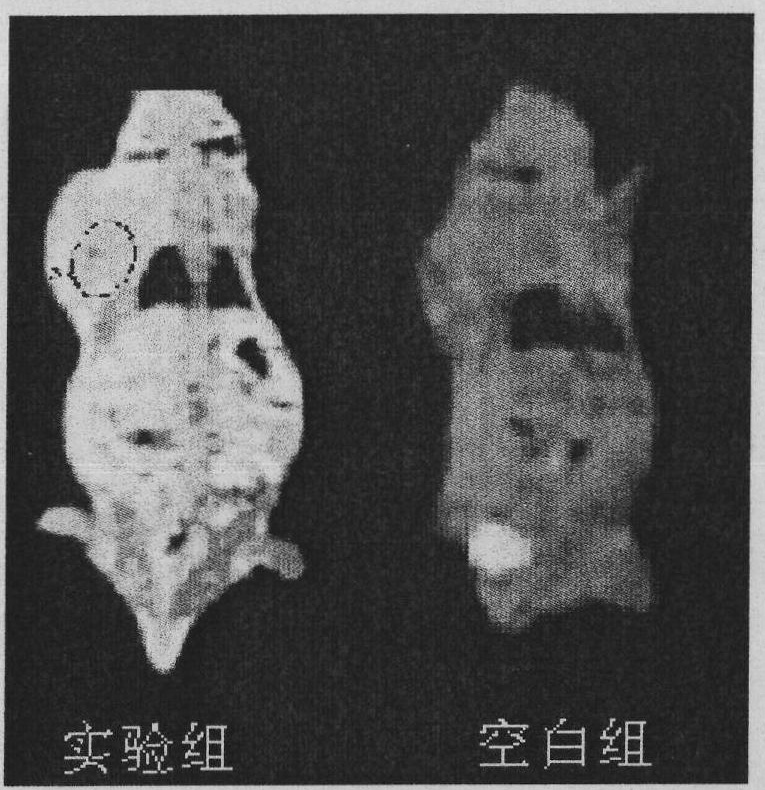
[0044] (2) Weigh 0.6g of the porphyrin compound modified by diethylenetriaminepentaacetic acid and dissolve it in 50ml of water, add 0.54g of gadolinium chloride, adjust the pH to 7.0-8.0, stir electromagnetically for 12h, and pour the reaction solution into 30mL (CH 3 CH 2 OH: (CH 3 CH 2 ) 2 (0=1: 1) separate out product in the mixed solution, suction filtration, obtain the porphyrin modified by ga...
Embodiment 2
[0048] The preparation method of the porphyrin modified by gadolinium diethylene triamine pentaacetate comprises the steps:
[0049] (1) 0.5g 5-(4-aminophenyl)-10,15,20-phenylporphyrin, 1.2g diethylenetriaminepentaacetic acid bicyclic anhydride, 0.5ml triethylamine were dissolved in 20mL pyridine, Under the catalysis of 4,4-dimethylaminopyridine, it was stirred at room temperature for 36h. After the reaction is complete, add 30mL of H 2 O, precipitated product. Suction filtration to obtain the crude product. Washing with dichloromethane for 3 times, ethanol for 3 times, drying, and recrystallization to obtain a porphyrin compound modified with diethylenetriaminepentaacetic acid;
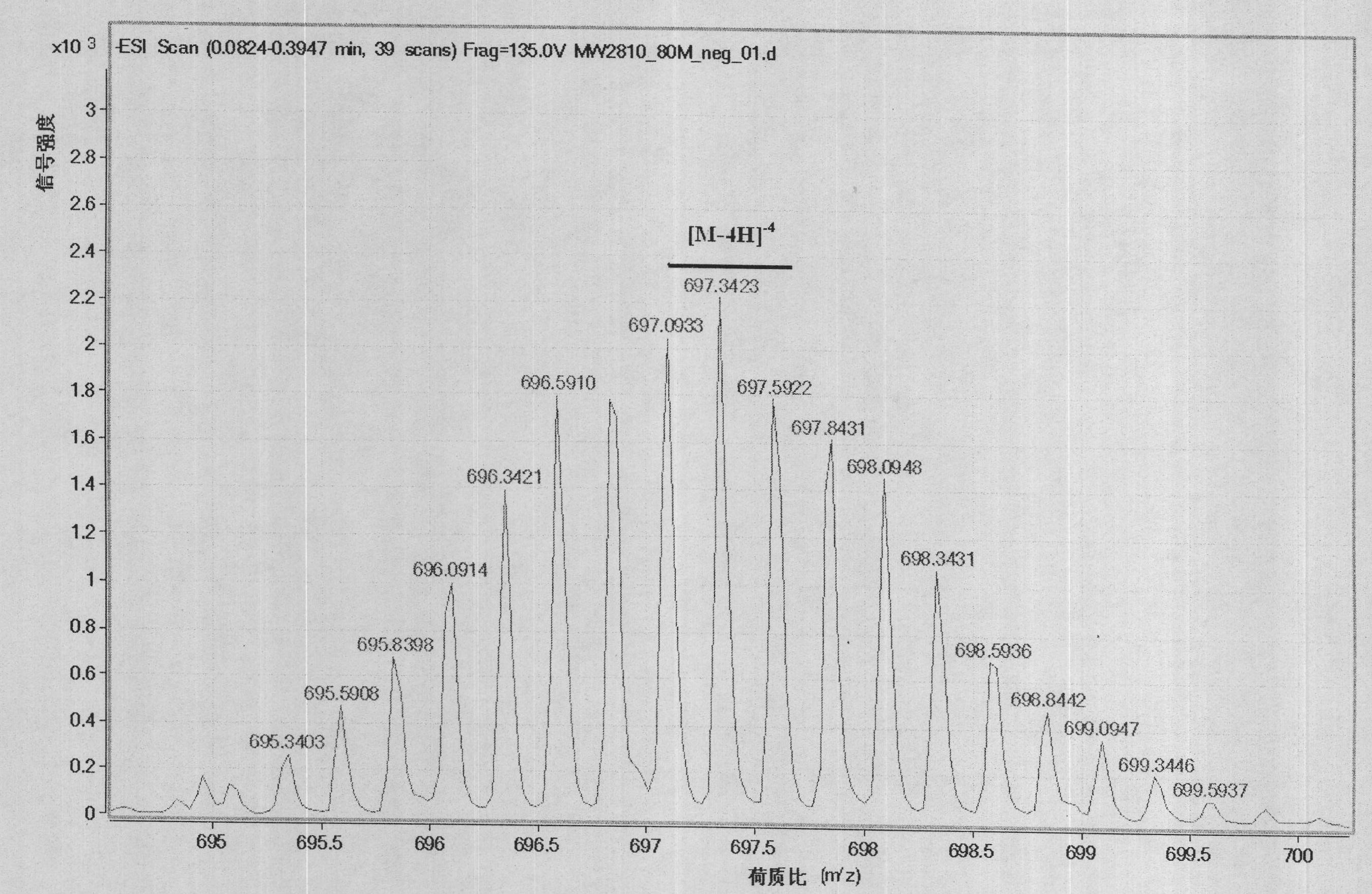
[0050] (2) Dissolve 0.3 g of the diethylenetriaminepentaacetic acid-modified porphyrin compound in 30 ml of water, add 0.29 g of gadolinium chloride, adjust the pH to 7.0-8.0, and stir electromagnetically for 20 h. 8mL of water was evaporated, and the reaction solution was poured into 30mL (CH3 C...
Embodiment 3
[0053] The preparation method of the porphyrin modified by gadolinium diethylene triamine pentaacetate comprises the steps:
[0054] (1) 0.5g 5,10-bis(4-aminophenyl)-15,20-diphenylporphyrin, 2.0g diethylenetriaminepentaacetic acid bicyclic anhydride, 2.0ml triethylamine are dissolved in N, In 30 mL of N-dimethylformamide, under the catalysis of 4,4-dimethylaminopyridine, stir at room temperature for 36 h. After the reaction is complete, add 30mL of H 2 0, the product is separated out, suction filtered, washed 3 times with 6ml methylene chloride, washed 3 times with 10ml ethanol, and recrystallized to obtain the porphyrin compound modified by diethylenetriaminepentaacetic acid;
[0055] (2) Weigh 0.6g of diethylenetriaminepentaacetic acid-modified porphyrin compound and dissolve it in 50ml of water, add 0.68g of gadolinium chloride, adjust the pH to 7.0-8.0, and react with electromagnetic stirring for 30h. 10mL of water was evaporated, and the reaction solution was poured int...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com