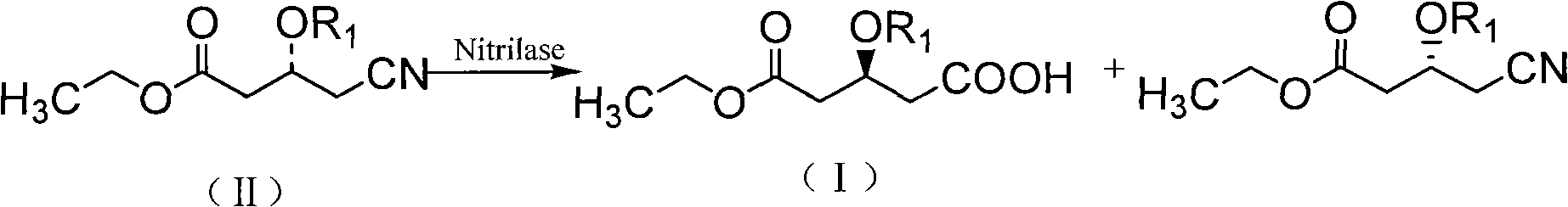
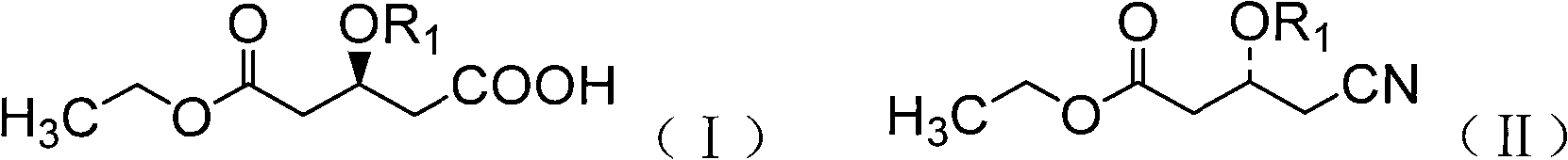Biological catalysis method for preparing statin medicinal intermediate
A technology of biocatalysis and intermediates, applied in biochemical equipment and methods, microorganism-based methods, microorganisms, etc., can solve the problems of difficult synthesis, difficult screening of biocatalysts, and no nitrilase strains.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
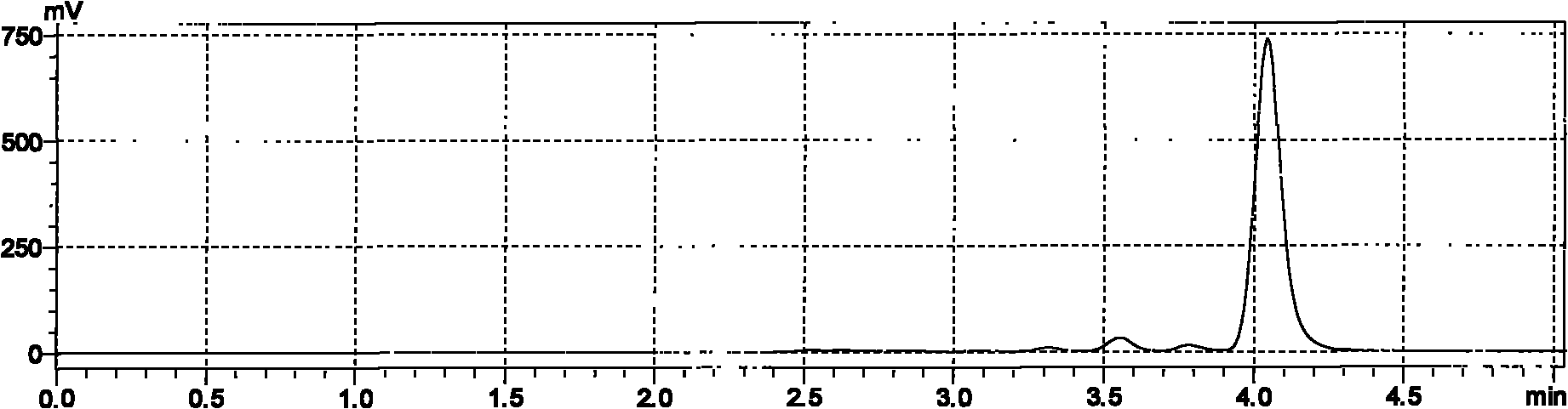
[0038] Example 1: Using Co 2+ Screening of nitrilase strains capable of selectively catalyzing the hydrolysis of ethyl 4-cyano-3-hydroxybutyrate to ethyl (R)-3-hydroxyglutarate by ion chromatography
[0039] (1) Prepare 1% soil sample suspension and sewage suspension with sterile water, inoculate 5ml to 45ml enrichment medium respectively, and culture on a shaker at 30°C and 150rpm for 2 days. The composition of each liter of enrichment medium is: glucose 5g, K 2 HPO 4 0.5g, MgSO 4 ·7H 2 O 0.5g, NaCl 0.1g, FeSO 4 ·7H 2 O 0.02g, solvent is water, pH 6.5. After aliquoting, sterilize at 0.1 MPa for 20 minutes, and add sterile-filtered ethyl 4-cyano-3-hydroxybutyrate to 1% (v / v) before inoculation. After the cultivation, take the turbid culture solution and inoculate 1ml into 49ml enrichment medium respectively, culture on a shaker at 30°C and 150rpm for 2 days, and enrich in this way 4 times. Take the relatively well-growing culture solution to coat the separation plate,...
Embodiment 2
[0044] Example 2: Preparation of biocatalyst R. erythropolis CCTCC NO: M209244 cells
[0045] Pick a ring of bacteria from the inclined surface of the test tube of the new nitrilase-producing strain R.erythropolis CCTCCNO: M 209244 obtained by the present invention, inoculate it into 50ml sterile seed medium, and place it on a shaker at 30°C and 150rpm Cultivate for 24 hours to obtain seed solution. The composition of each liter of seed medium is: glucose 8g, peptone 5g, K 2 HPO 4 0.5g, MgSO 4 ·7H 2 O 0.2g, the solvent is water, the pH is natural, and sterilized at 0.1MPa for 20min.
[0046] The seed solution was transferred to 100.0ml sterile fermentation medium with 3% (v / v) inoculation amount, and cultured on a shaker at 30°C and 150rpm for 48h. The composition of each liter of fermentation medium is: glucose 20g, peptone 10g, caprolactam 1g, K 2 HPO 4 0.5g, MgSO 4 ·7H 2 O 0.2g, solvent is water, pH 7.0. Sterilize at 0.1MPa for 20min. After cultivating, obtain ...
Embodiment 3
[0047] Example 3: Preparation of biocatalyst R. erythropolis CCTCC NO: M 209149 cells The preparation method of the whole cell catalyst is the same as in Example 2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com