Preparation method of hydrogen selenide
A technology of hydrogen selenide and zinc selenide, applied in the direction of binary selenium/tellurium compounds, etc., can solve problems such as environmental pollution and large equipment investment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
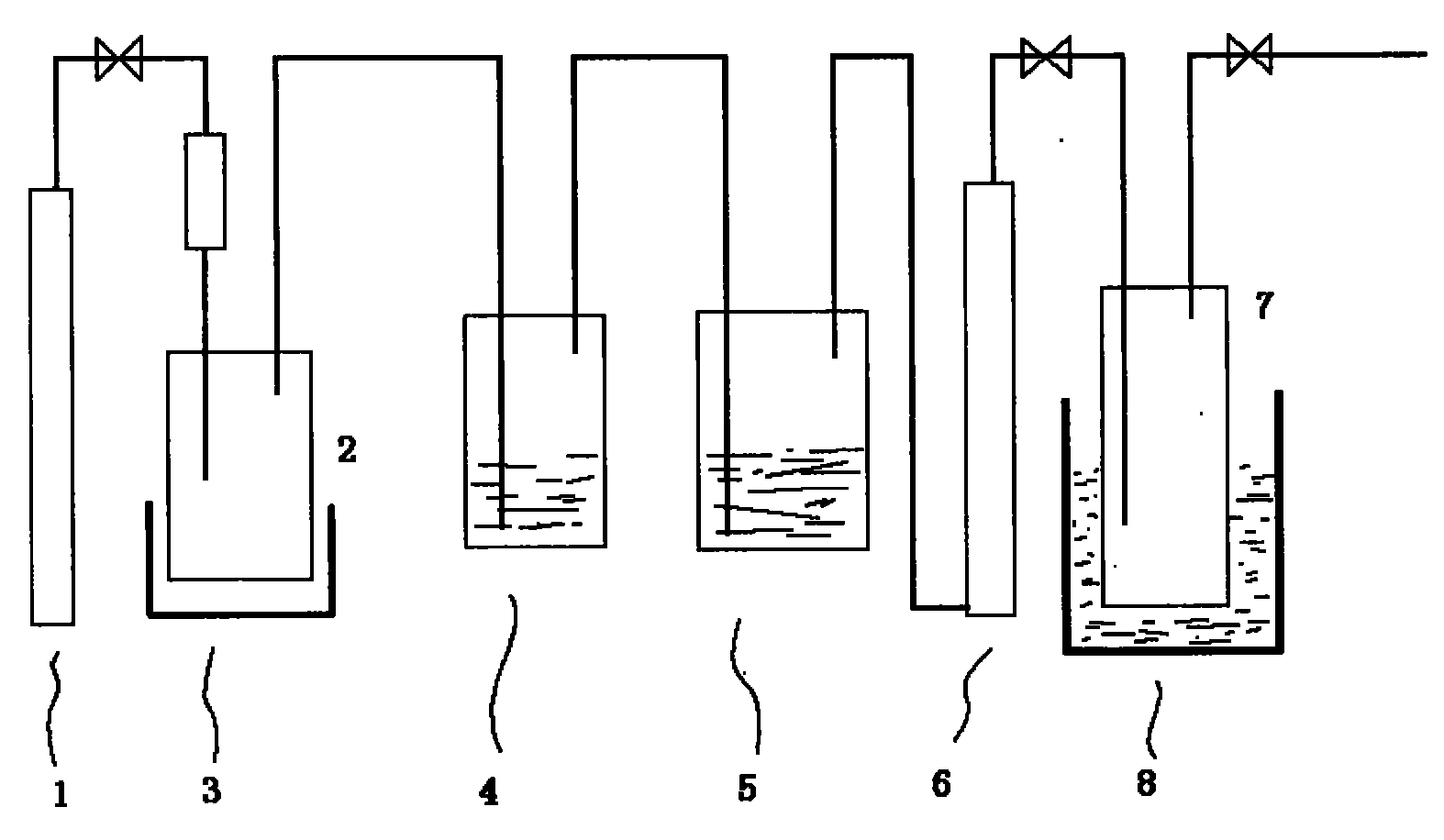
[0034] 10 grams of zinc selenide is added in the reactor 2, and the whole system is purged with high-purity nitrogen to remove the air in the system; the reactor 2 is heated to 80°C with the heater 3, and 20 milliliters of 30% concentrated hydrochloric acid is added dropwise, one After hours of dropwise addition, the gas enters the receiving bottle 7 after being processed by the washer 4, the water washer 5 and the dryer 6, and the receiving bottle 7 is soaked in the cold well 8, and the temperature of the cold well is controlled at -110°C. The purity of hydrogen selenide was 95% (volume fraction) by gas chromatography, and the yield was 76%. After the reaction was finished, 5 milliliters of hydrogen peroxide was added dropwise to the liquid in the reactor 2 to remove hydrogen selenide in the liquid.
Embodiment 2
[0036] 10 grams of zinc selenide was added to reactor 2, and the whole system was purged with high-purity nitrogen to remove the air in the system; reactor 2 was heated to 80°C with heater 3, and 30 ml of 30% sulfuric acid was added dropwise for one hour After the dropwise addition, the gas is processed by the washer 4, the water washer 5 and the drier 6 and enters the receiving bottle 7. The receiving bottle 7 is soaked in the cold well 8, and the temperature of the cold well is controlled at -100°C. The purity of hydrogen selenide was 95% (volume fraction) as checked by gas chromatography, and the yield was 70%. After the reaction was finished, 5 milliliters of hydrogen peroxide was added dropwise to the liquid in the reactor 2 to remove hydrogen selenide in the liquid.
Embodiment 3
[0038] 500 grams of zinc selenide was added to reactor 2, and the air in the system was removed with high-purity nitrogen. Heat the reactor 2 to 80°C with the heater 3, start to drop 1000 milliliters of 30% concentrated hydrochloric acid, control the reaction process, the dropwise addition is completed in about 3 hours, and the generated gas passes through the washer 4, the water washer 5 and the drier 6 After the treatment, it enters the receiving bottle 7, and the receiving bottle 7 is soaked in the cold well 8, and the temperature of the cold well is controlled at -80°C. Obtain 205 grams of hydrogen selenide, the purity is greater than 95% (volume fraction), and the yield is 80%. After the reaction was finished, 20 milliliters of hydrogen peroxide was added dropwise to the liquid in the reactor 2 to remove hydrogen selenide in the liquid.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com