Isosorbide mononitrate freeze-dried power injection and preparation method thereof
A technology of isosorbide dinitrate and freeze-dried powder injection is applied to the field of freeze-dried powder injection of isosorbide mononitrate and its preparation, which can solve the problem that the safety of the stabilizer is not fully guaranteed, the hydrolysis of the drug is unavoidable, and the product is stable. Good reconstitution, improved drug safety, and stable content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
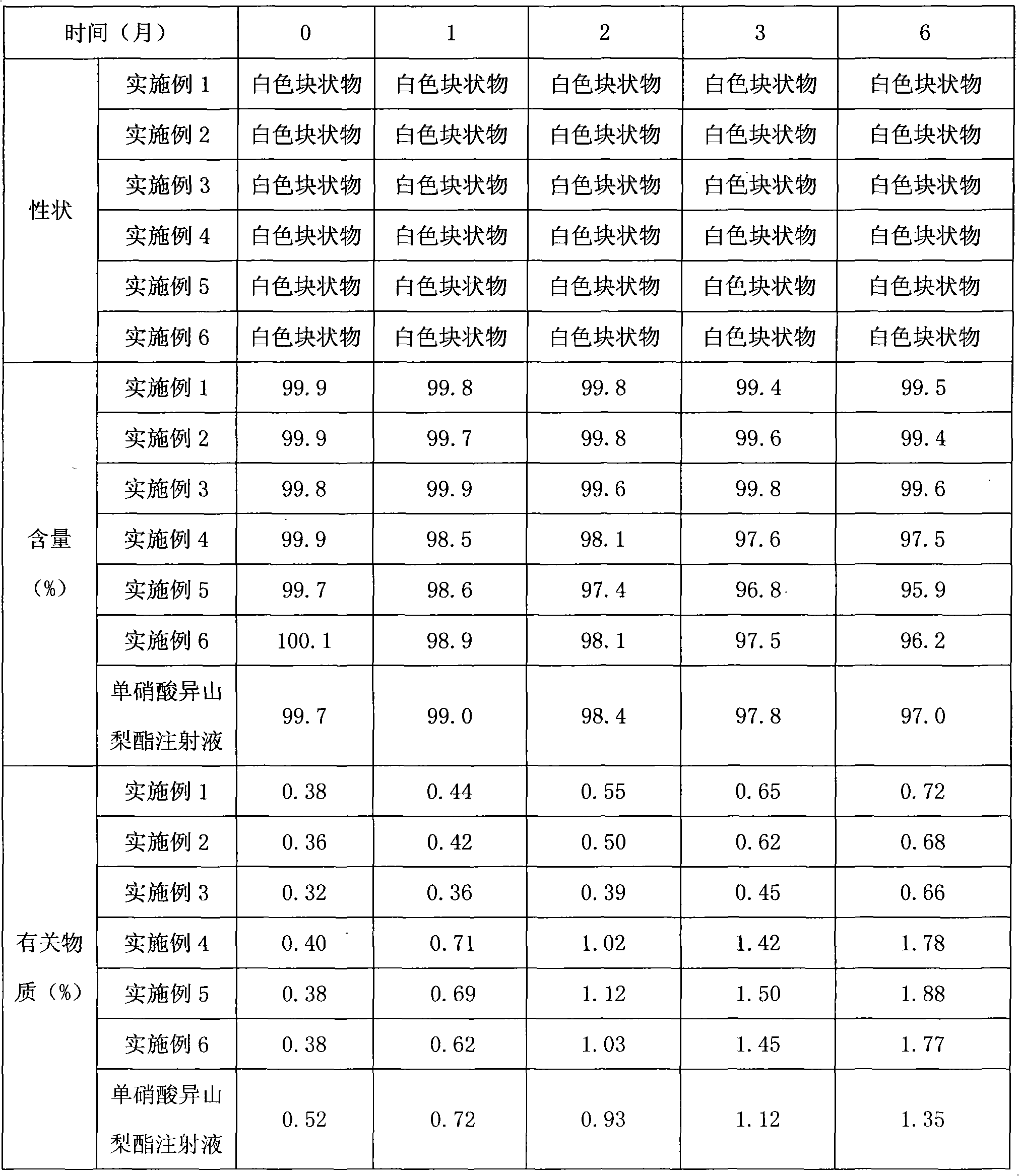
Embodiment 1
[0037] 1. Prescription composition:
[0038] Isosorbide Mononitrate 20g
[0039] Sorbitol 40g
[0040] Phosphate buffer (pH 6.5) 100ml
[0041] Add sterile water for injection to 2000ml
[0042]
[0043] Freeze-dried to make a total of 1000 bottles
[0044] 2. Preparation method:
[0045] (1) Preparation: get 1600ml water for injection and add sorbitol to make it dissolve completely, add gac, absorb at normal temperature for 30min, filter to remove charcoal, add the isosorbide mononitrate of the prescription amount under room temperature, then add the phosphate buffer saline of pH 6.5 ( Take 0.68g of potassium dihydrogen phosphate, add 15.2ml of 0.1mol / L sodium hydroxide solution, dilute to 100ml with water) 100ml, add water for injection to the full amount to obtain an intermediate solution, and filter to sterilize with a 0.22um sterile microporous membrane;
[0046] (2) Check the clarity of the ...
Embodiment 2
[0050] 1. Prescription composition:
[0051] Isosorbide Mononitrate 20g
[0052] Sorbitol 100g
[0053] Phosphate buffer (pH 7.5) 200ml
[0054] Add sterile water for injection to 2000ml
[0055]
[0056] Freeze-dried to make a total of 1000 bottles
[0057] 2. Preparation method:
[0058] (1) Preparation: Take 1600ml of water for injection and add excipients to dissolve it completely, add activated carbon, absorb at room temperature for 30 minutes, filter to remove carbon, add the prescribed amount of isosorbide mononitrate at room temperature, and then add phosphate buffer with pH 6.6 (Take 1.74g of potassium dihydrogen phosphate, 2.7g of disodium hydrogen phosphate and 1.7g of sodium chloride, add water to dissolve to 400ml) 200ml, add water for injection to the full amount to obtain an intermediate solution, filter and sterilize with a 0.22um sterile microporous membrane ;
[0059] (2) Check ...
Embodiment 3
[0063] 1. Prescription composition:
[0064] Isosorbide Mononitrate 20g
[0065] Sorbitol 80g
[0066] Phosphate buffer (pH 7.0) 100ml
[0067] Add sterile water for injection to 2000ml
[0068]
[0069] Freeze-dried to make a total of 1000 bottles
[0070] 2. Preparation method:
[0071] (1) Preparation: get 1600ml water for injection and add sorbitol to make it dissolve completely, add gac, absorb at room temperature for 30min, filter to remove charcoal, add prescription amount of isosorbide mononitrate at room temperature, then add the phosphate buffer saline of pH 7.0 ( Take 0.68g of potassium dihydrogen phosphate, add 29.1ml of 0.1mol / L sodium hydroxide solution, add water to dilute to 100ml) 100ml, add water for injection to the full amount to obtain an intermediate solution, and filter to sterilize with a 0.22um sterile microporous membrane;
[0072] (2) Check the clarity of the i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com