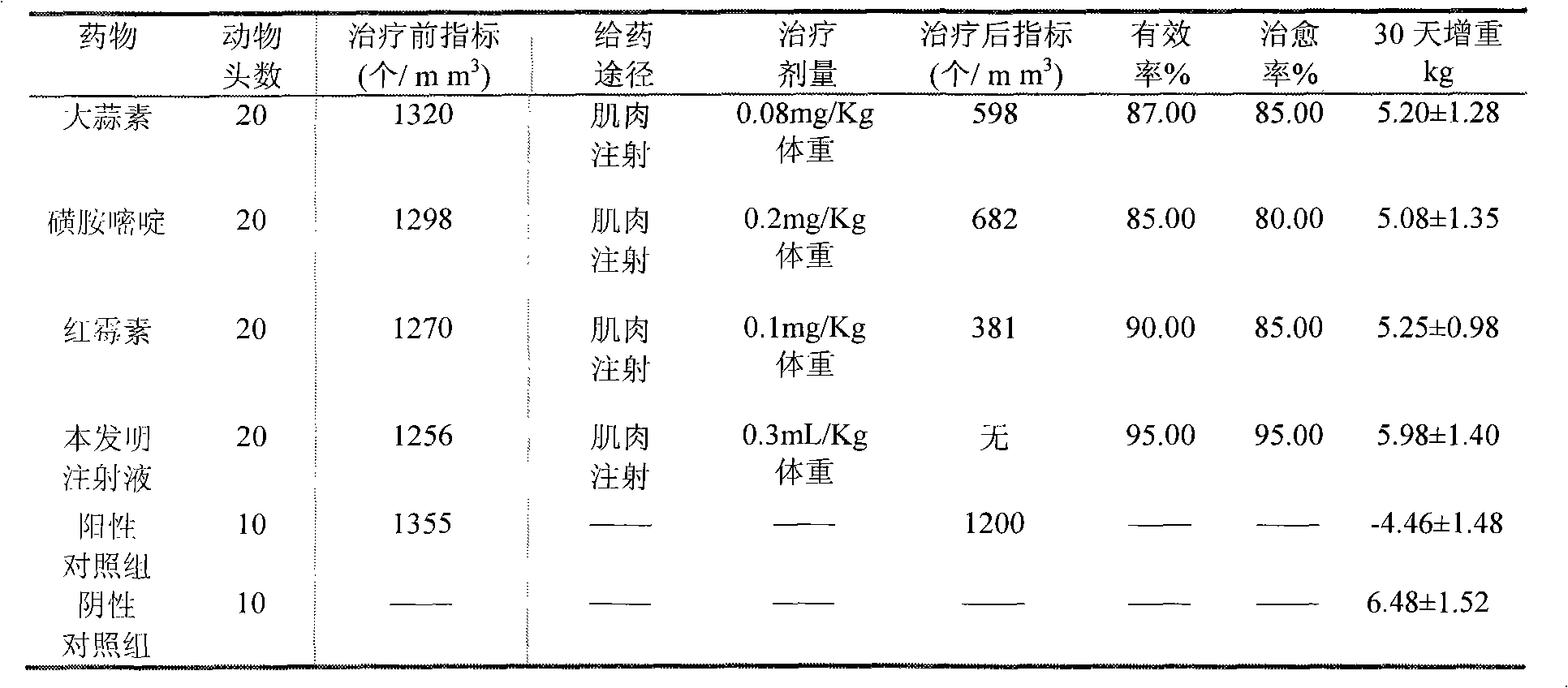
Medicinal composition and injecta for curing avian toxoplasmosis, and preparation method thereof
A technology of toxoplasmosis and composition, which is applied in the field of veterinary drugs, can solve the problems of large toxic and side effects, unsatisfactory curative effect, and inability to cure, and achieve the effects of low toxic and side effects, alleviating high fever symptoms, and preventing secondary infection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] 1. The pharmaceutical composition for the treatment of livestock toxoplasmosis consists of the following components by weight percentage:
[0045] Sulfamethoxine 18%
[0046] Sulfamethoxazole 55%
[0047] Trimethoprim 7%
[0048] Pyrimethamine 14%
[0049] Diclofenac Sodium 6%.
[0050] 2. The injection for the treatment of livestock toxoplasmosis consists of the following components by weight percentage:
[0051] Sulfamethoxine 5%
[0052] Sulfamethoxazole 15%
[0053] Trimethoprim 2%
[0054] Pyrimethamine 3%
[0055] Diclofenac Sodium 1.5%
[0056] Polyethylene Glycol-400 5%
[0057] Propylene Glycol 20%
[0058] Alpha-Pyrrolidone 45%
[0060] Disodium EDTA 0.01%
[0061] The balance is water for injection.
[0062] 3. The preparation method of the injection for the treatment of livestock toxoplasmosis is carried out according to the following steps:
[0063] (1) Heat the mixed solution of polyethylene glycol-400, propylene...
Embodiment 2
[0067] 1. The pharmaceutical composition for the treatment of livestock toxoplasmosis consists of the following components by weight percentage:
[0068] Sulfamethoxine 20%
[0069] Sulfamethoxazole 52%
[0070] Trimethoprim 8%
[0071] Pyrimethamine 13%
[0072] Aspirin 7%.
[0073] 2. The injection for the treatment of livestock toxoplasmosis consists of the following components by weight percentage:
[0074] Sulfamethoxine 5.5%
[0075] Sulfamethoxazole 14.5%
[0076] Trimethoprim 2.2%
[0077] Pyrimethamine 3%
[0078] Aspirin 2%
[0079] Polyethylene glycol-400 6%
[0080] Propylene Glycol 22%
[0081] Alpha-Pyrrolidone 35%
[0082] Sodium bisulfite 0.11%
[0083] Disodium EDTA 0.02%
[0084] The balance is water for injection.
[0085] 3. The preparation method of the injection for the treatment of livestock toxoplasmosis is carried out according to the following steps:
[0086] (1) Heat the mixed solution of polyethylene glycol-400, propylene glycol and α...
Embodiment 3
[0090] 1. The pharmaceutical composition for the treatment of livestock toxoplasmosis consists of the following components by weight percentage:
[0091] Sulfamethoxine 22%
[0092] Sulfamethoxazole 55%
[0093] Trimethoprim 9%
[0094] Pyrimethamine 6%
[0095] Magnesium Salicylate 8%.
[0096] 2. The injection for the treatment of livestock toxoplasmosis consists of the following components by weight percentage:
[0097] Sulfamethoxine 6%
[0098] Sulfamethoxazole 15%
[0099] Trimethoprim 2.5%
[0100] Pyrimethamine 1.6%
[0101] Magnesium Salicylate 2.2%
[0102] Polyethylene glycol-400 7%
[0103] Propylene Glycol 24%
[0104] Alpha-Pyrrolidone 40%
[0105] Sodium metabisulfite 0.12%
[0106] Disodium EDTA 0.02%
[0107] The balance is water for injection.
[0108] 3. The preparation method of the injection for the treatment of livestock toxoplasmosis is carried out according to the following steps:
[0109] (1) Heat the mixed solution of polyethylene glyco...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com