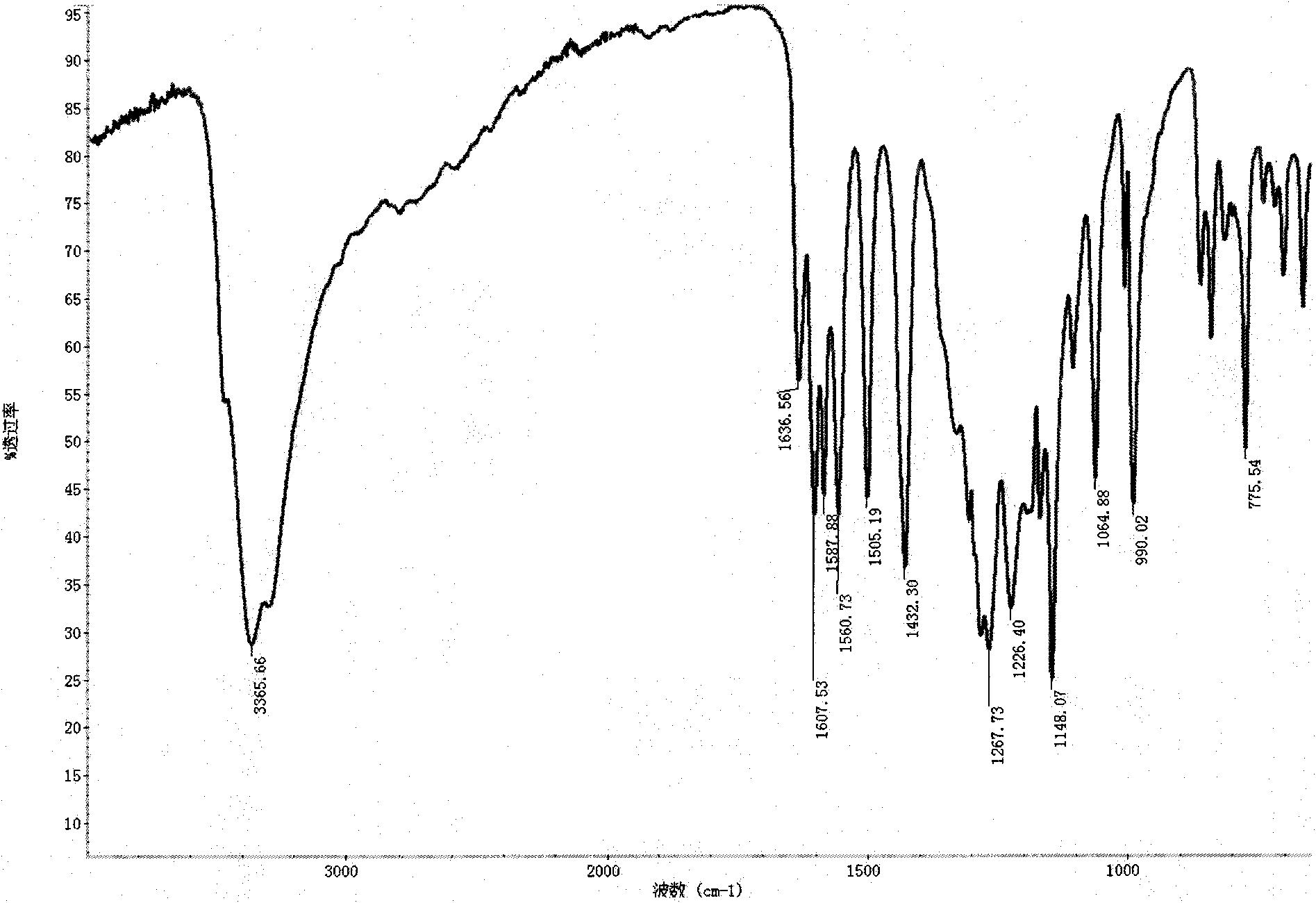
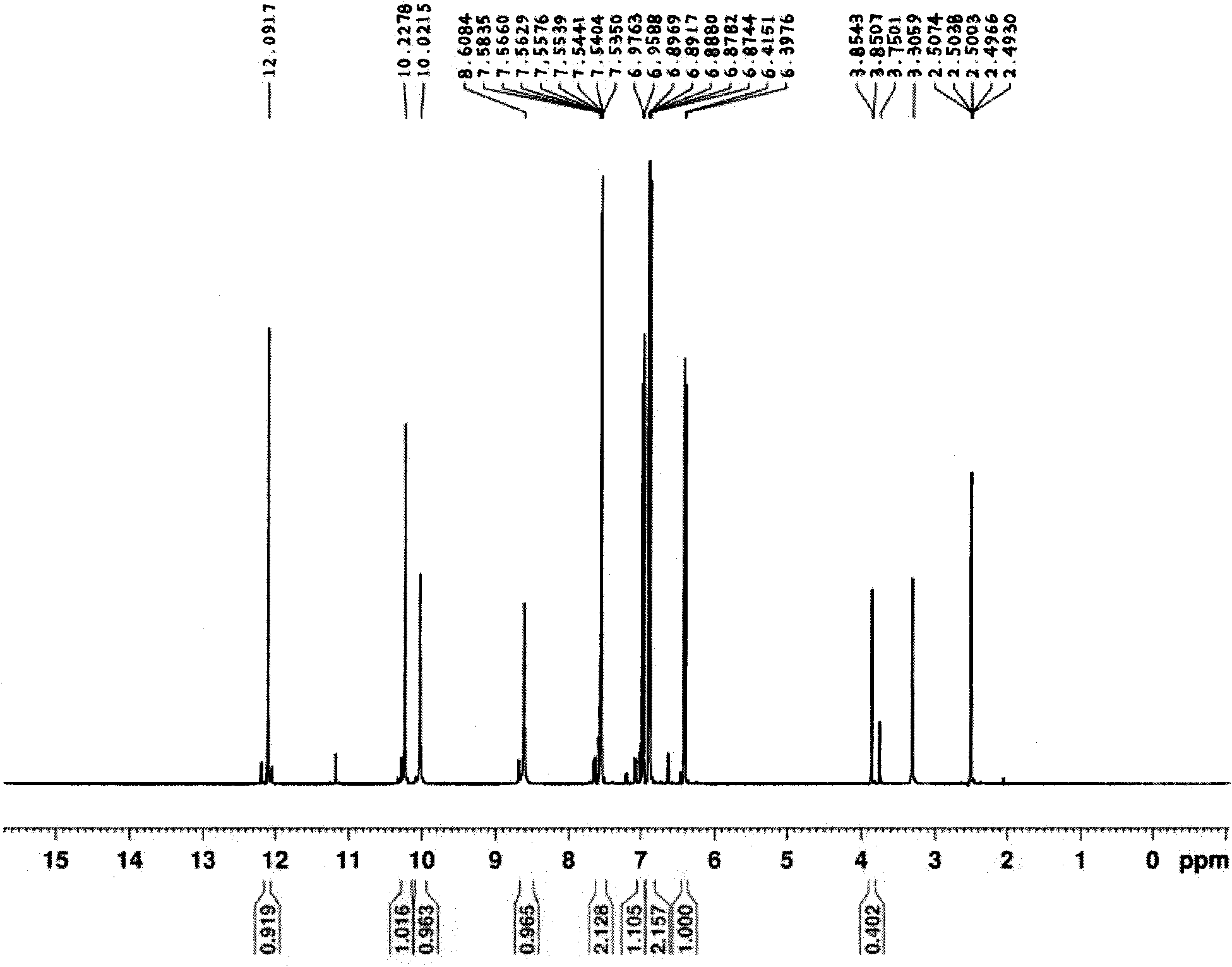
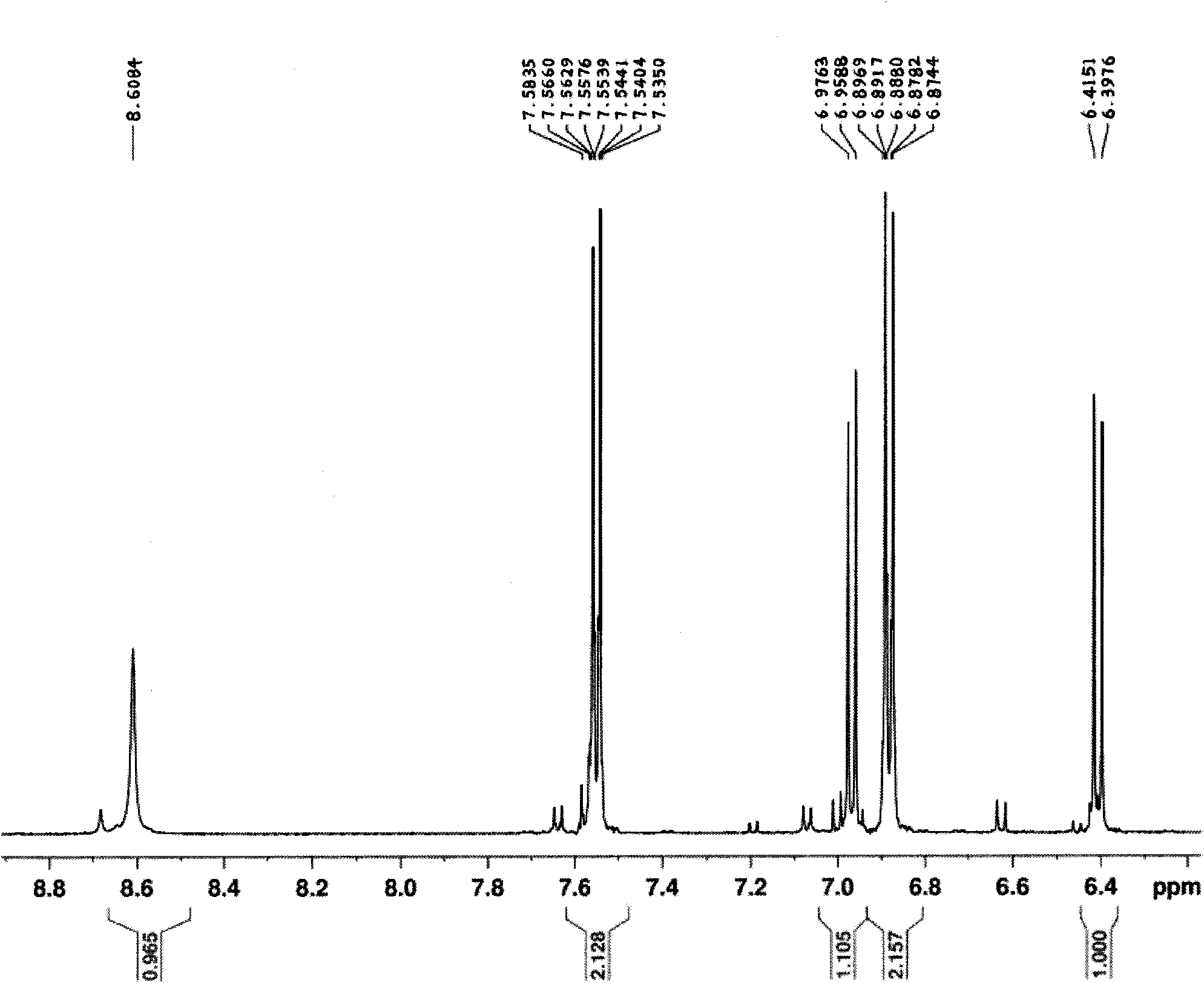
Method for synthesizing 2,3,4,4'-tetrahydroxybenzophenone
A technology of tetrahydroxybenzophenone and p-hydroxybenzoic acid, which is applied in the direction of chemical instruments and methods, condensation preparation of carbonyl compounds, organic compound/hydride/coordination complex catalysts, etc., can solve the problem of large amount of waste liquid, reaction Long time and other problems, to achieve the effect of short reaction time, reduce preparation cost, and reduce the amount of waste liquid
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] A process for synthesizing 2,3,4,4'-tetrahydroxybenzophenone, the steps are:
[0024] (1) According to the mass ratio of pyrogallic acid and p-hydroxybenzoic acid as 1: 1.1 (mol: mol), pyrogallic acid and p-hydroxybenzoic acid are acylated under a certain catalyst and temperature, and the reaction ends After cooling, the reaction solution was neutralized with alkaline solution to precipitate crystals, filtered, washed with water and dried to obtain a crude product of 2,3,4,4'-tetrahydroxybenzophenone. The catalyst used in the acylation reaction is boron trifluoride methanol solution, the ratio of the amount to the quality of pyrogallic acid is 4:1 (mL:g); the lye is 3-5% (g / mL) sodium bicarbonate solution , the amount of lye used is to control the pH value of the solution to a neutral limit; the reaction temperature is 100-120°C; the reaction time is 2-6h.
[0025] (2) Dissolve the crude product of 2,3,4,4'-tetrahydroxybenzophenone obtained by the reaction in boiling w...
Embodiment 2
[0027] (1) Weigh 6.3g of pyrogallic acid and 7.6g of p-hydroxybenzoic acid, put them into a 500mL three-necked reaction flask, add 25mL of methanol solution of boron trifluoride as a catalyst, and react at 110°C for 6h, cool after the reaction, and the reaction solution Neutralize with 3% (g / mL) sodium bicarbonate solution, adjust the pH value to neutral, precipitate crystals, filter, wash with water and dry to obtain the crude product of 2,3,4,4'-tetrahydroxybenzophenone 10.06 g.
[0028] (2) The above crude product was dissolved in 500mL of boiling water, added 1.5g of activated carbon for decolorization for 20min, filtered while hot, the filtrate was cooled and crystallized, filtered, washed with pure water, dried to obtain 2,3,4,4'-tetrahydroxybenzidine Ketone refined product 7.72g.
Embodiment 3
[0030] Weigh 6.3g of pyrogallic acid and 7.6g of p-hydroxybenzoic acid, put them into a 500mL three-necked reaction flask, add 25mL of methanol solution of boron trifluoride as catalyst, react at 120°C for 2h, cool after the reaction, and use 5% (g / mL) sodium bicarbonate solution to neutralize, adjust the pH value to neutral, precipitate crystals, filter, wash with water and dry to obtain 9.1 g of crude product of 2,3,4,4'-tetrahydroxybenzophenone.
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| flash point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com