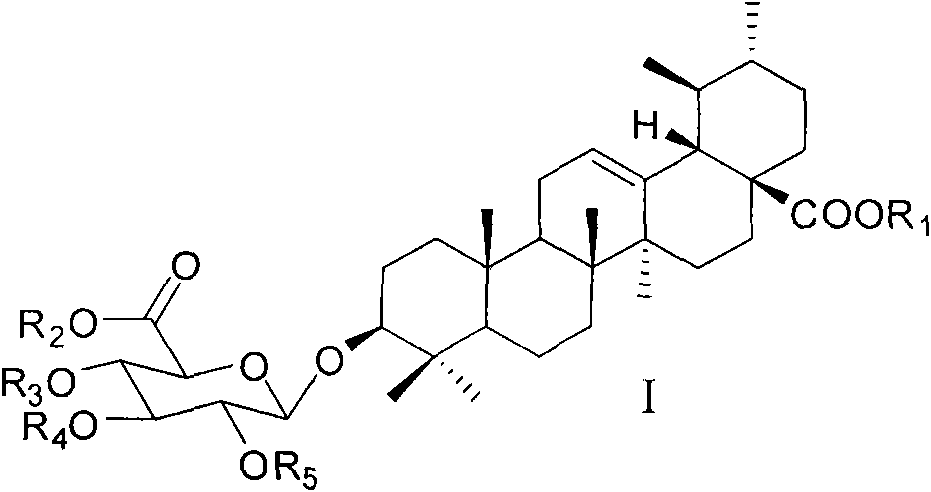
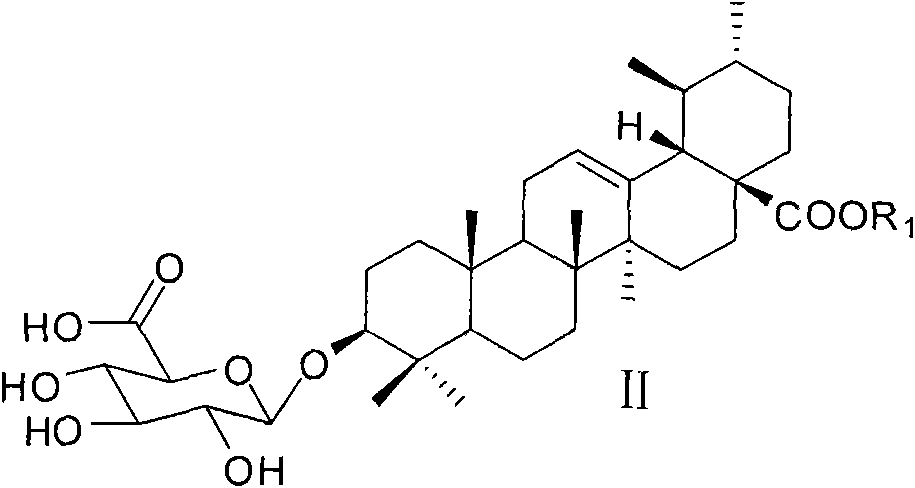
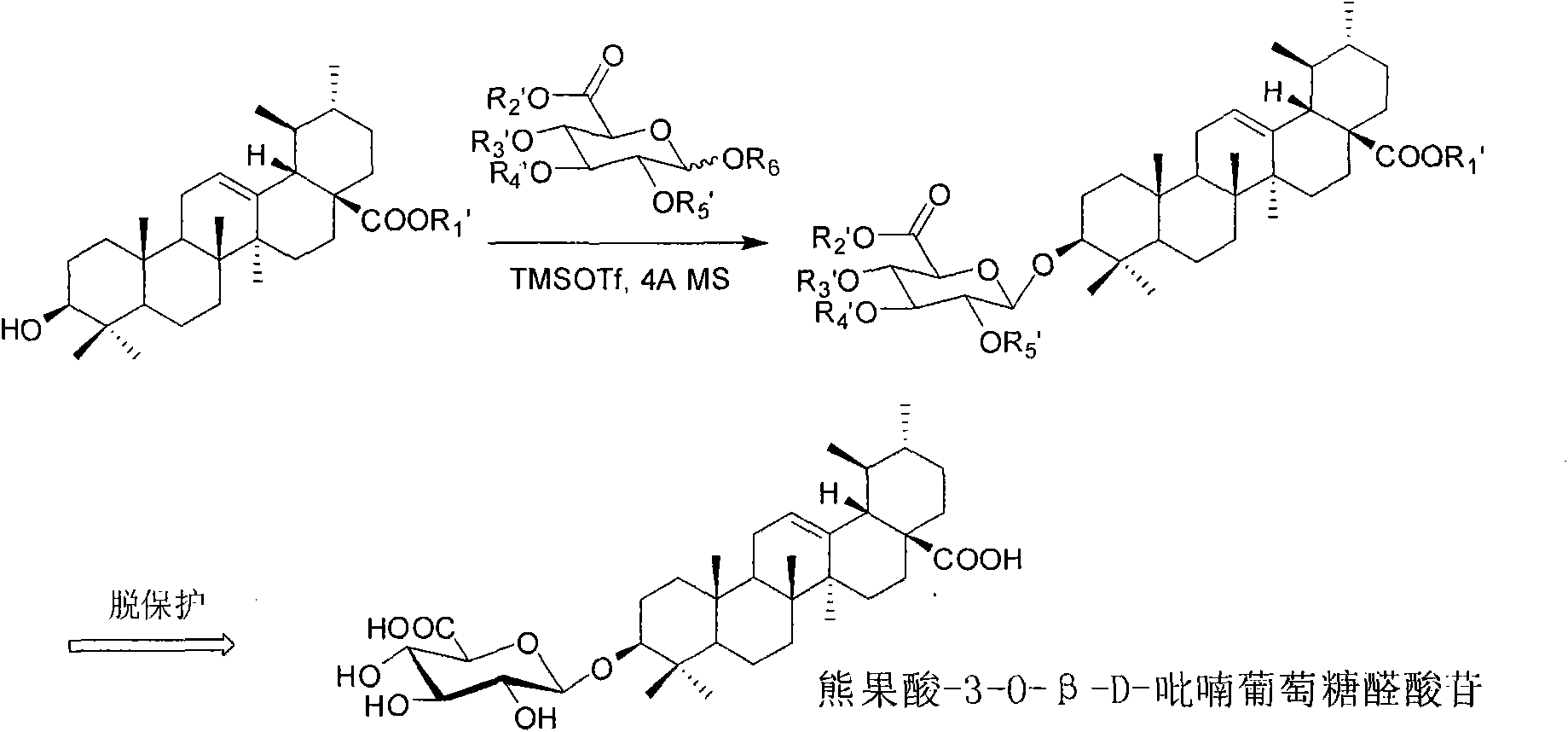
Ursolic acid-3-O-beta-D-pyranglucuronide and derivatives thereof, and preparation method and medicinal application thereof
A technology of glucopyranose and ursolic acid is applied in the field of medicine to achieve the effects of reducing absorption, good preventive and therapeutic effects, and reducing postprandial hyperglycemia
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Synthesis of ursolic acid-28-benzyl ester-3-O-β-D-(2,3,4-tribenzoyloxyglucuronomethyl)glycoside
[0053] Benzyl ursolic acid (400mg, 0.73mmol) and imidate compound (1.3eq) were dissolved in 15mL of dry dichloromethane, added Molecular sieve 3g, the suspension was stirred in an ice bath for 30 min, then a dry dichloromethane solution of TMSOTf (0.0276M, 0.4 mL) was added, the reaction solution was reacted in an ice bath for 40 min, and triethylamine was added to quench the reaction. After filtering the reaction solution, the filtrate was evaporated to dryness and directly subjected to silica gel column chromatography to obtain ursolic acid-28-benzyl ester-3-O-β-D-(2,3,4-tribenzoyloxyglucuronide methyl ester). ESI-MS m / z: 1071.53[M+Na] + ; 1 H NMR (CDCl 3 , 300MHz) δ0.60(s, 3H), 0.62(s, 3H), 0.70(s, 3H), 0.86(s, 6H), 0.94(s, 3H), 1.03(s, 3H), 2.25(d , J=12.1Hz, 1H), 3.13-3.18(m, 1H), 3.70(s, 3H), 4.31(d, J=9.8Hz, 1H), 4.89(d, J=7.8Hz, 1H), 4.96 and 5.09(d, J=12.4Hz...
Embodiment 2
[0055] Synthesis of ursolic acid-3-O-β-D-(2,3,4-tribenzoyloxyglucuronomethyl)glycoside
[0056] Dissolve ursolic acid-28-benzyl ester-3-O-β-D-(methyl 2,3,4-tribenzoyloxyglucuronate) (100mg, 0.1mmol) in THF 3mL, add Catalytic amount of palladium carbon, the reaction solution quantitatively removes the 28-position benzyl group under a hydrogen environment to obtain ursolic acid-3-O-β-D-(2,3,4-tribenzoyloxyglucuronic acid methyl ester) glycosides. ESI-MS m / z: 981.48[M+Na] + ; 1 HNMR (CDCl 3 )δ0.60(s, 3H), 0.71(s, 3H), 0.72(s, 3H), 0.86(s, 3H), 0.88(s, 3H), 0.95(s, 3H), 1.04(s, 3H ), 2.15-2.19(m, 1H), 3.13-3.18(m, 1H), 3.70(s, 3H), 4.31(d, J=9.8Hz, 1H), 4.89(d, J=7.78Hz, 1H) , 5.22(brs, 1H), 5.56-5.69(m, 2H), 5.91(t, J=9.6Hz, 1H), 7.26-7.96(m, 15H); 13 C NMR (CDCl 3 )δ15.4, 16.2, 17.0, 18.1, 21.2, 23.3, 23.5, 24.2, 27.8, 28.0, 29.7, 30.6, 33.0, 36.7, 38.7, 38.9, 39.1, 39.5, 42.0, 47.6, 48.0, 52.6, 52.8, 55.5 , 70.5, 71.9, 72.5, 72.9, 90.8, 103.3, 126.0, 128.3, 128.4, 128.9...
Embodiment 3
[0058] Synthesis of Ursolic Acid-3-O-β-D-Glucopyranoside
[0059] Dissolve ursolic acid-3-O-β-D-(2,3,4-tribenzoyloxyglucuronide methyl ester) glycoside (80 mg, 0.13 mmol) in 5 mL of dry methanol, add a catalytic amount of Sodium methoxide, stirred at room temperature for 40 minutes, cooled the reaction solution to 0°C in an ice bath, added 1 mL of 2 mol / L sodium hydroxide aqueous solution dropwise, and continued to stir the reaction solution at room temperature for 3 hours, then neutralized the reaction solution to acidity with acetic acid, After the reaction solution was evaporated to dryness under reduced pressure, it was subjected to silica gel column chromatography to obtain ursolic acid-3-O-β-D-glucopyranose. ESI-MS m / z: 631.5[M-H] - ; 1 H NMR (C 5 D. 5 N) δ0.72(s, 3H), 0.90(s, 6H), 0.93(s, 6H), 1.20(s, 3H), 1.23(s, 3H), 2.55(d, J=11.3Hz, 1H) , 3.30(m, 1H), 3.96-4.01(m, 1H), 4.22(brs, 1H), 4.49(brs, 1H), 4.91(brs, 1H), 5.39(brs, 1H); 13 C NMR (C 5 D. 5 N) δ15.6, 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com