New method for preparing LixFeyPzO4 by using ferro phosphorus
A new method, ferrophosphorus technology, applied in chemical instruments and methods, phosphorus compounds, inorganic chemistry, etc., can solve the problems of high efficiency and difficulty in precise control, and achieve high efficiency, short reaction process, clean and environmentally friendly production process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
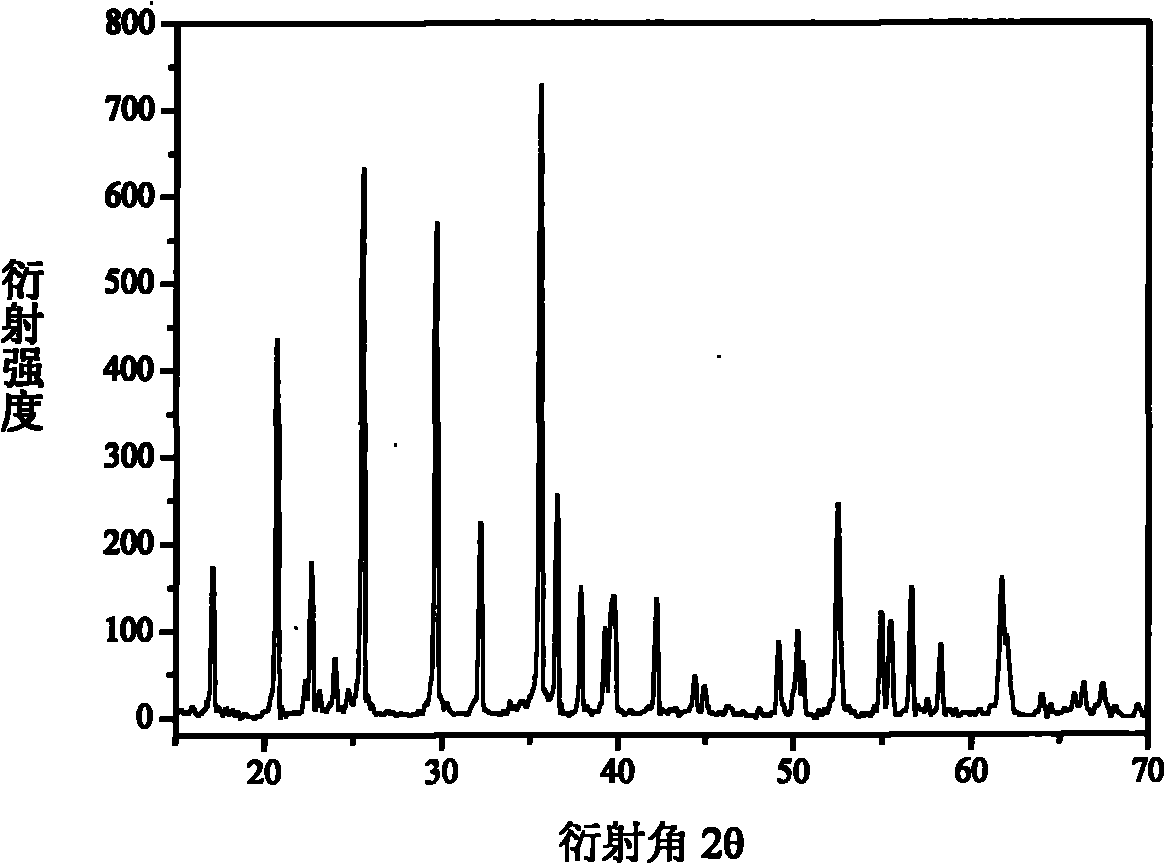
[0022] Ferrophosphorus FeP, Fe(CO) 5 , Li 3 PO 4 Preparation of LiFePO as raw material 4 , as Fe(CO) 5 As the precursor of the conductive agent, with P element as the balance agent, FeP, Fe(CO) 5 , P, Li 3 PO 4 Dosing according to the molar ratio of 7:8:3:5, mixed evenly, transferred to a closed tube furnace and roasted at 600-800°C for 15-25 hours to obtain carbon-doped LiFePO 4 , the reaction that occurs is as follows:
[0023] 7FeP+8Fe(CO) 5 +5Li 3 PO 4 +3P→15LiFePO 4 +40C
[0024] Fe(CO) 5 The raw material is obtained by the reaction of Fe and CO, and CO can be reduced to CO by C 2 , so that the greenhouse gas CO 2 Through this reaction, it is converted into C, reducing greenhouse gas emissions and realizing a low-carbon economy, and the C generated by the reaction is used as LiFePO 4 In-situ doping of conductive agent, no other by-products are generated, the whole process is clean and environmentally friendly, and the product has a better crystal form, such...
Embodiment 2
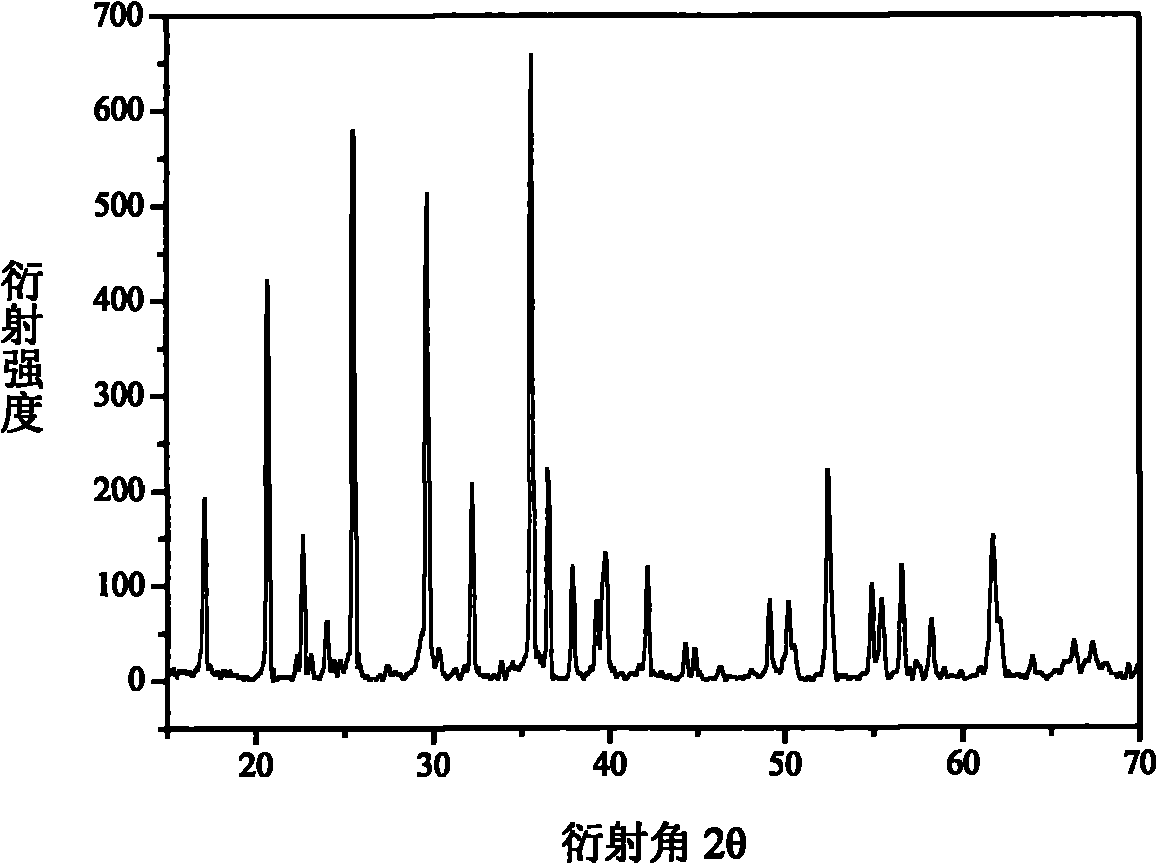
[0026] Fe 1.5 P, Fe 2 o 3 ,P 2 o 5 , Li 2 CO 3 Preparation of LiFePO as raw material 4 , the Fe 1.5 P, Fe 2 o 3 ,P 2 o 5 , Li 2 CO 3 Dosing according to the molar ratio of 4:16:17:19.05, with 5-10% glucose as the precursor of the conductive agent, mixed evenly, transferred to a closed reaction kettle with a pressure reducing valve and reacted at 600-800°C for 16- 25 hours, get LiFePO 4 , the reaction that occurs is as follows:
[0027] 4Fe 1.5 P+16Fe 2 o 3 +17P 2 o 5 +19Li 2 CO 3 →38LiFePO 4 +19CO 2 ↑
[0028] The separated CO 2 After the product is absorbed by LiOH solution, it can be concentrated and dried to make Li 2 CO 3 Raw materials can also be reacted with Fe after carbon reduction to produce Fe(CO) 5 , as the raw material of Example 1, no other by-products are generated, and green and clean process production is realized. Prepared LiFePO 4 The product is relatively loose and has a good olivine structure, such as figure 2 shown.
Embodiment 3
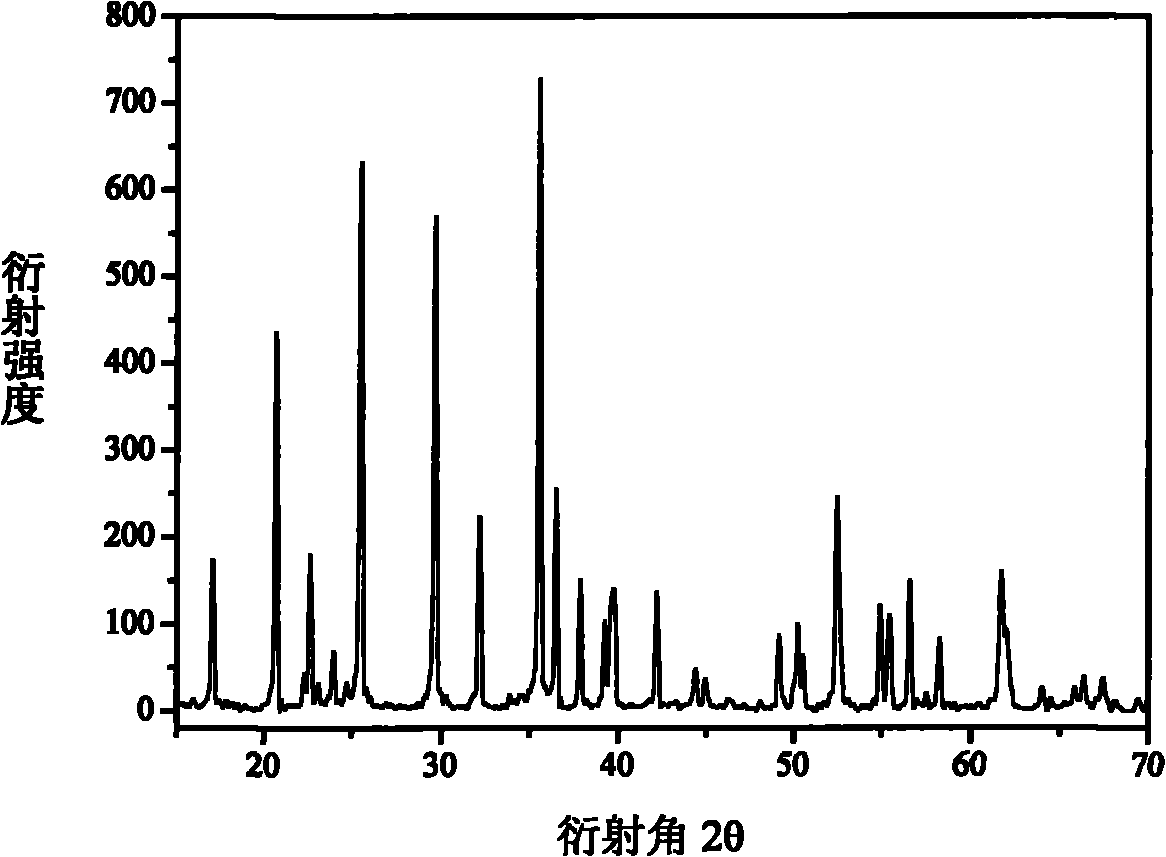
[0030] FeP 2 , Fe 2 o 3 ,P 2 o 5 , Li 2 o 2 Preparation of LiFePO as raw material 4 , with 5-10wt.% ferrophosphorus as the conductive agent, and Fe as the balancer, the FeP 2 , Fe, Fe 2 o 3 ,P 2 o 5 , Li 2 o 2 Dosing according to the molar ratio of 1:1:3:3:4, mixed evenly, milled with 500rpm high-energy ball for 1-5h, then transferred to a closed muffle furnace and roasted at 650-800°C for 5-10 hours to obtain LiFePO 4 , the reaction that occurs is as follows:
[0031] FeP 2 +Fe+3Fe 2 o 3 +3P 2 o 5 +4Li 2 o 2 →8LiFePO 4
[0032] No other by-products are produced in the reaction, and the whole production process is green, clean and environmentally friendly.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com