Fusion protein polymer
A technology of fusion protein and multimer, which is applied in the direction of hybrid peptide, introduction of foreign genetic material by using carrier, and cells modified by introducing foreign genetic material, which can solve the problems of low affinity and limited utilization, and achieve improved affinity and cost Low, high expression effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
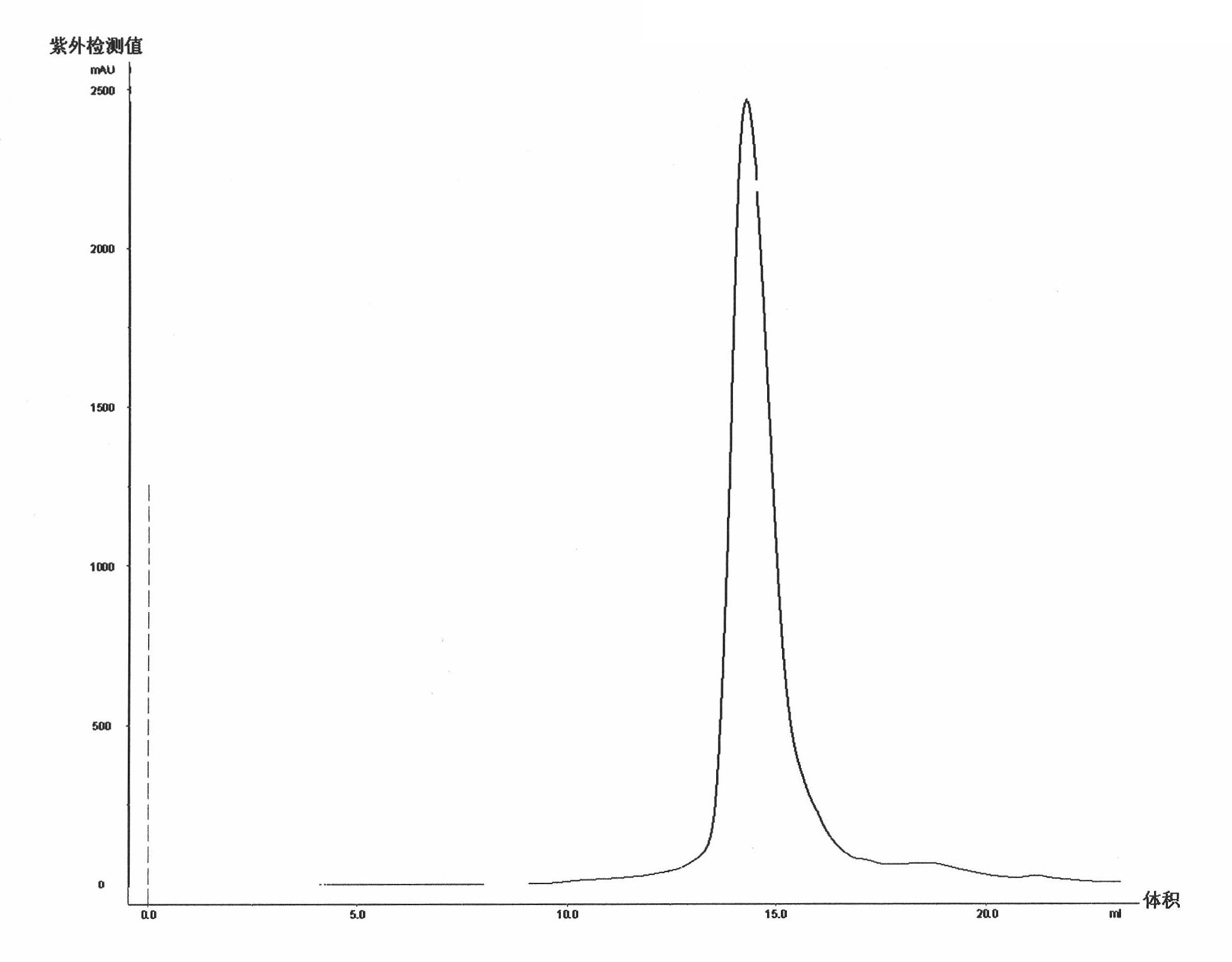
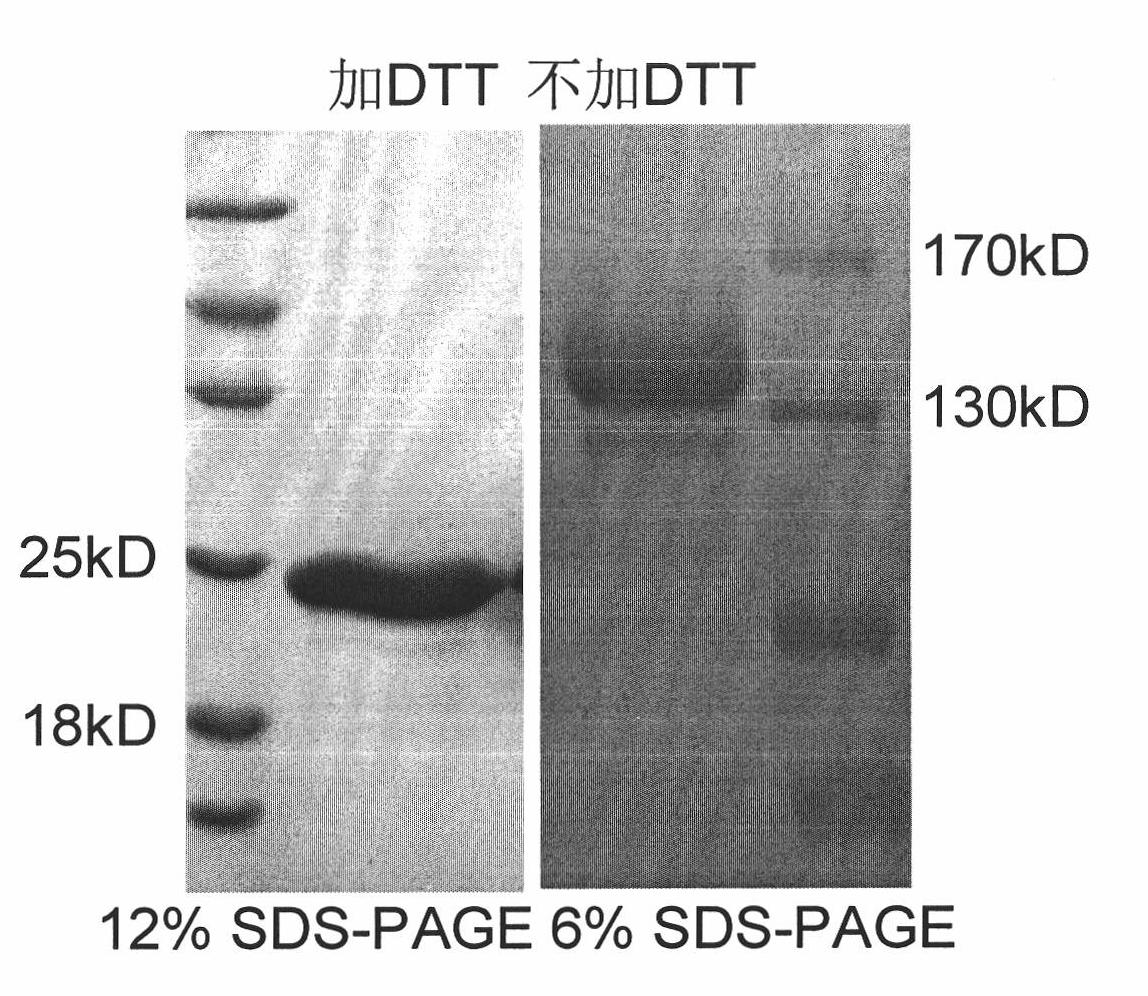
[0038] Example 1 Preparation and Verification of Single Region Antibody Pentameric Protein
[0039] 1. Clone MT1COMP and MT1S:
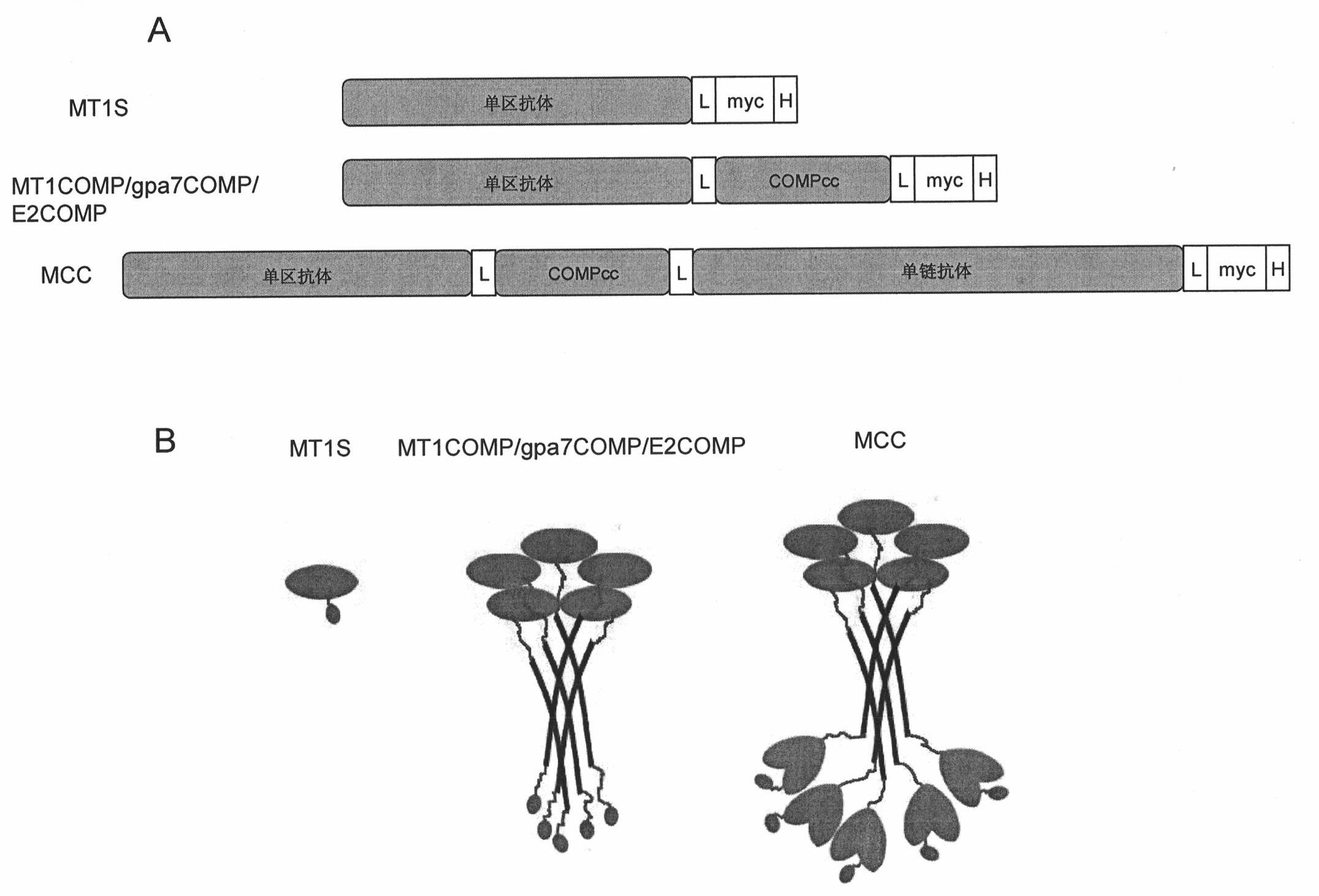
[0040] MT1 was fused with COMP to make a pentameric (method see figure 1 ), first use the PCR method to amplify the gene fragment expressing MT1, and the primers used are:
[0041] MT1F: 5′ TAATAAGAAGACCGCAGGCCGATGTGCAGCTGCAGGCGTCTGG 3′; sdAbR: 5′ ATTATTATGGGCCCTGAGGAGACGGTGACCTGGGT 3′
[0042] Template: phage expressing MT1 (Beijing Episource Biotechnology Co., Ltd.)
[0043] Reaction system: 10×pfu buffer 10μl, pfu 1μl, dNTP 8μl, MT1F 20μl, sdAbR 20μl, template 1μl, H 2 O 40 μl.
[0044] Reaction program: 94°C 5min; 94°C 30s, 60°C, 30s, 72°C 40s, 30 cycles; 72°C 10min
[0045] There are Bbs I and Apa I restriction sites at both ends, and after digestion and digestion, pVT2 digested with Bbs I and Apa I (Zhang et al., J Mol Biol, 335, 2004, 49-56) was inserted to obtain plasmid MT1VT1B. Then PCR amplifies the COMP fragment, and the primers use...
Embodiment 2
[0061] Antigen specificity experiment of embodiment 2MT1COMP pentamer fusion protein
[0062] Antigen specificity of MT1COMP was analyzed by ELISA.
[0063] 1) Preparation of biotinylated bovine serum albumin and HLA-A2 complex:
[0064] Biotinylated bovine serum albumin (BSA-biotin) was obtained by labeling BSA with sulfo-NHS-LC-Biotin (pierce, Rockford), and the specific operation was strictly in accordance with the instructions provided by the manufacturer. Biotinylated HLA-A2 complexes were prepared according to the relevant steps in the preparation of HLA-A2 complex tetramers by Dirk Busch (http: / / www.mikrobio.med.tu-muenchen.de / category / laboratory-group -busch / ) (the method is as follows: first express HLA-A2 and β in Escherichia coli 2 Inclusion bodies of m (HLA-A2 and β 2 The m gene is provided by Beijing Table Source), after purification, with different polypeptides (HLA-A2 restriction, including: MART-1 (ELAGIGILTV), gp100 (IMDQVPESV), CMV (NLVPMVATV), FLU (GILGFV...
Embodiment 3
[0073] Example 3 Affinity experiment of MT1COMP pentameric fusion protein
[0074] Adopt surface plasmon resonance (surface plasmon resonance, SPR) technique to measure MT1COMP pentamer fusion protein, the affinity of MT1S, detection equipment is Biacore 3000 (Biacore AB company, Sweden), used chip is Sensor chipSA (BiacoreAB company, Sweden), The chip was prepared according to the instructions provided by the manufacturer, and PBST was used as the running buffer throughout the process. Add the biotinylated MART-1-HLA-A2 complex, and at the same time add the biotinylated gp100-HLA-A2 complex to another channel to calculate the background during detection. See Figure 5 for the fixed density of the complex illustrate. MT1S set five concentration gradients 0.125μM, 0.25μM, 0.5μM, 1μM, 2μM, flowing through the chip from low to high, between the two concentrations with 10mM borate buffer (PH8.5, containing 1M NaOH and 0.1% Tween-20) regenerates the surface of the HLA-A2 complex. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com