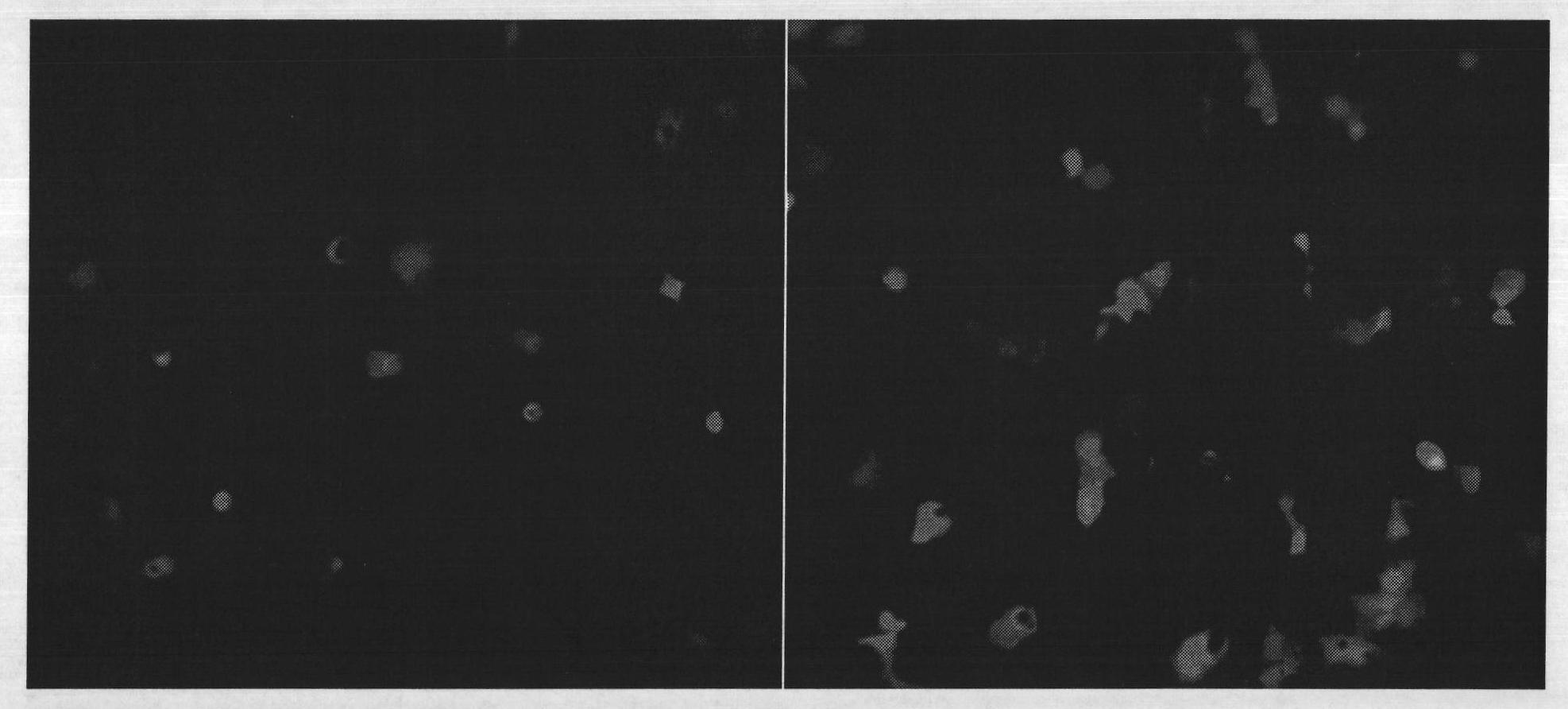
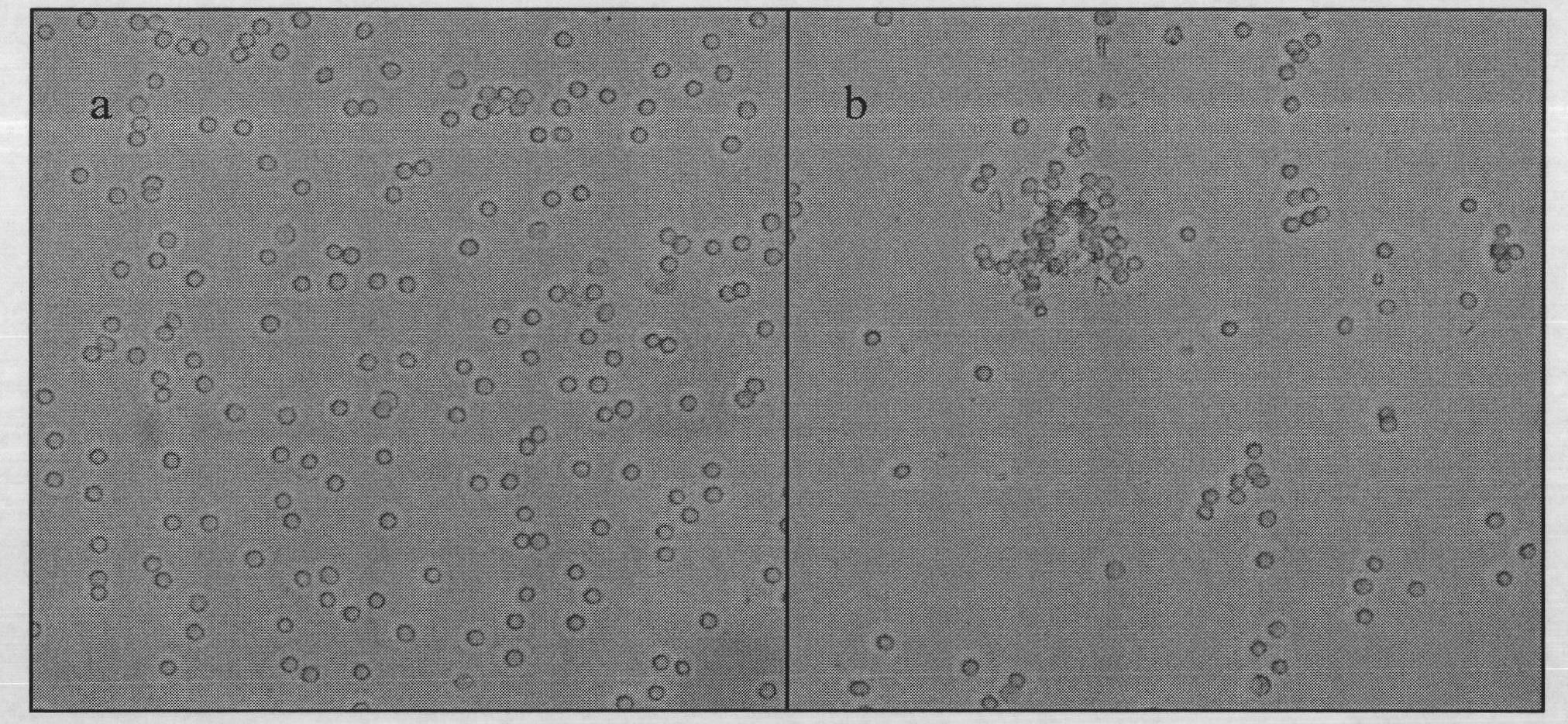
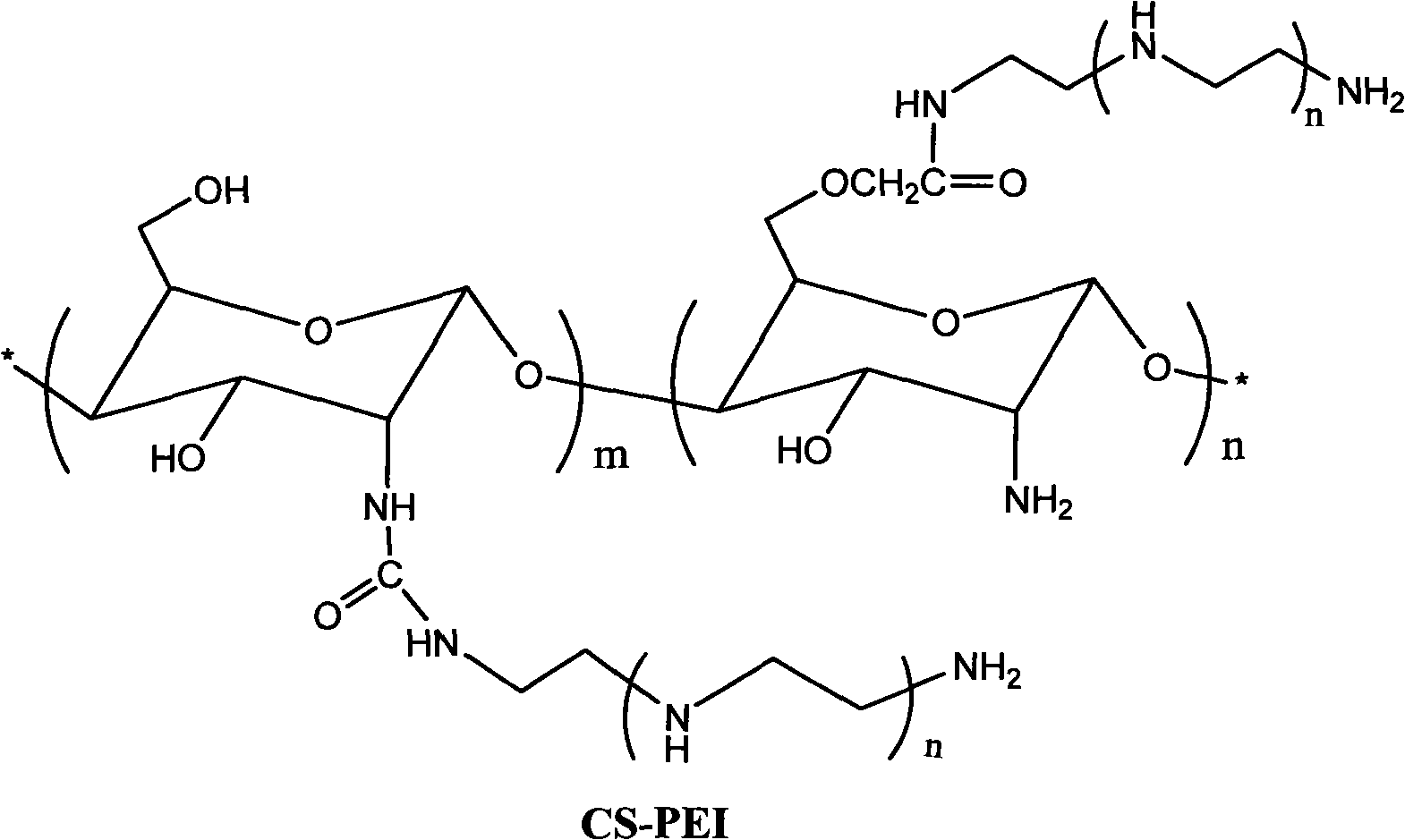
PEI (Polyetherimide)-chitosan triply compound gene vector with low molecular weight and preparation method and application thereof
A technology of gene carrier and chitosan, which is applied in the fields of low molecular weight PEI-chitosan triple composite gene carrier and its preparation and application, can solve the problems of unstable transfection efficiency and low efficiency of composite particles, and prolong the circulation time in vivo. , mild conditions, reduce the effect of adsorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Add 32 ml of 40% NaOH solution into the beaker, add 3.2 g of chitosan (Mw=50 kDa, 0.02 mol) and soak in the beaker overnight. Pour off the lye in the upper layer, add 13ml of isopropanol, and then add chloroacetic acid (12.8g, 0.13mol), leave it at room temperature for 2h, then continue to react at 65°C for 3h, and cool down. Add 100ml of water to dissolve the reaction product, centrifuge at 10000r / min for 5min, discard the insoluble matter, and take the upper layer solution. Add excess ethanol to precipitate a white flocculent solid, filter the precipitate, add 50ml of water to dissolve, and dissolve the precipitate several times until the pH is neutral. The precipitate was put into a 37 degree oven for drying to obtain carboxymethyl chitosan.
[0028] Get 0.22g carboxymethyl chitosan (about 1mmol) in the there-necked flask, add 15ml water to dissolve, add 4gPEI (Mw=800, 5mmol), adjust the pH value to 5.0 with 38% hydrochloric acid, then add NHS (0.117g , 1 mmol), stir...
Embodiment 2
[0032] Add 32 ml of 40% NaOH solution into the beaker, add 3.2 g of chitosan (Mw=50 kDa, 0.02 mol) and soak in the beaker overnight. Pour off the lye in the upper layer, add 13ml of isopropanol, and then add chloroacetic acid (12.8g, 0.13mol), leave it at room temperature for 2h, then continue to react at 65°C for 3h, and cool down. Add 100ml of water to dissolve the reaction product, centrifuge at 10000r / min for 5min, discard the insoluble matter, and take the upper layer solution. Add excess ethanol to precipitate a white flocculent solid, filter the precipitate, add 50ml of water to dissolve, and dissolve the precipitate several times until the pH is neutral. The precipitate was put into a 37 degree oven for drying to obtain carboxymethyl chitosan.
[0033] Get 0.22g carboxymethyl chitosan (about 1mmol) in the there-necked flask, add 15ml water to dissolve, add 4g PEI (Mw=800), adjust the pH value to 5.0 with 38% hydrochloric acid, then add NHS (0.117g, 1 mmol), stirred a...
Embodiment 3
[0036] Add 32 ml of 40% NaOH solution into the beaker, add 3.2 g of chitosan (Mw=50 kDa, 0.02 mol) and soak in the beaker overnight. Pour off the lye in the upper layer, add 13ml of isopropanol, and then add chloroacetic acid (12.8g, 0.13mol), leave it at room temperature for 2h, then continue to react at 65°C for 3h, and cool down. Add 100ml of water to dissolve the reaction product, centrifuge at 10000r / min for 5min, discard the insoluble matter, and take the upper layer solution. Add excess ethanol to precipitate a white flocculent solid, filter the precipitate, add 50ml of water to dissolve, and dissolve the precipitate several times until the pH is neutral. The precipitate was put into a 37 degree oven for drying to obtain carboxymethyl chitosan.
[0037] Get 0.22g carboxymethyl chitosan (about 1mmol) in the there-necked flask, add 15ml water to dissolve, add 4gPEI (Mw=800), adjust pH value to 5.0 with 38% hydrochloric acid, then add NHS (0.117g, 1mmol ), stirred at roo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com