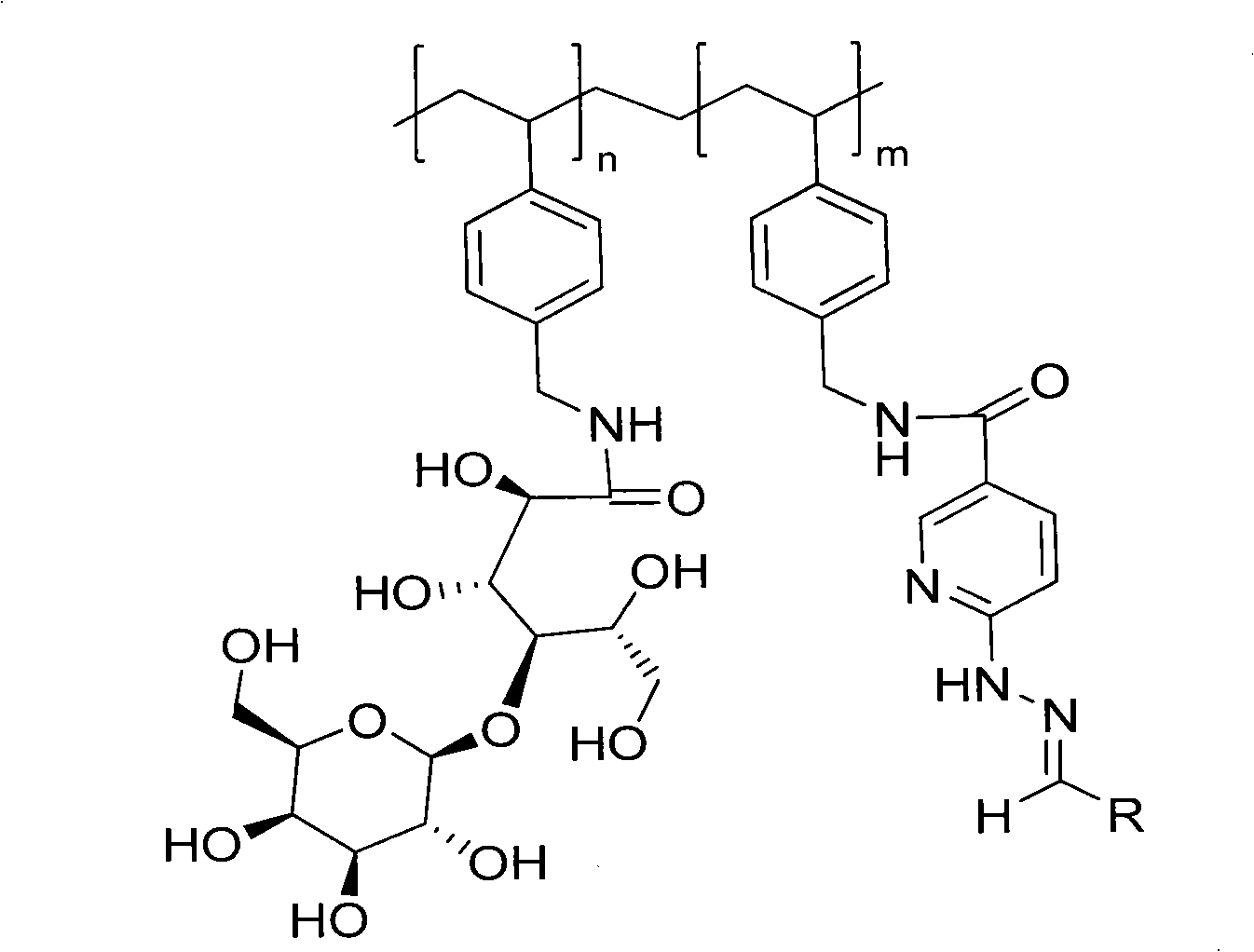
Technetium-99m-marked poly N-vinyl benzyl-D-lactose amide composition and preparation method
A technology of vinylbenzyl and lactamide, which is applied in the fields of radiopharmaceutical chemistry and clinical nuclear medicine, can solve the problems of human serum albumin being easily deteriorated and difficult to store.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
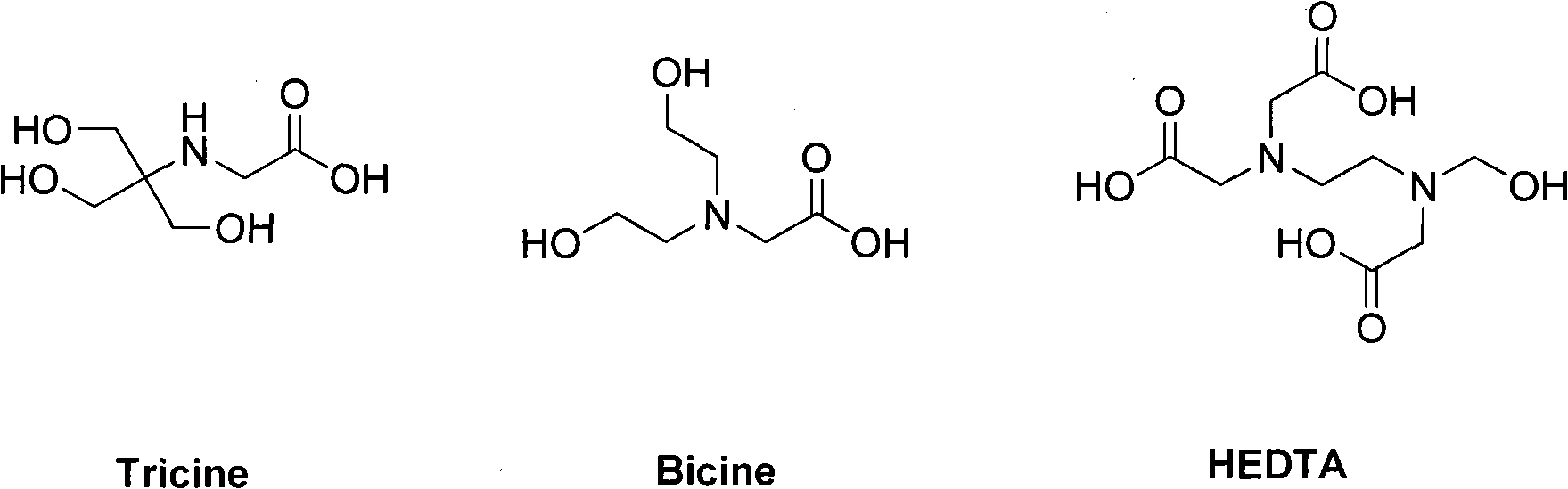
[0077] With Tricine as co-ligand, 99m Tc(PVLA)(tricine) 2 Preparation of:
[0078] Dissolve 0.2mg of p(VLA-co-VNI) in 0.5mL phosphate buffer (0.05mol / L pH6.0, containing 30mg co-ligand tricine), and add 2mg / mL SnCl 2 2H 2 O solution 10μL, shake well. Add fresh at the end 99m TCO 4 - Eluent 0.5mL (37MBq), fully shake, seal and react in boiling water bath for 20min. get 99m Tc(PVLA)(tricine) 2 .
[0079] The HiTrap desalting gel column (Sephadex G25) was equilibrated with 25 mL eluent (0.05 mol / L phosphate buffer at pH 7.5), and the flow rate was controlled between 1 and 10 mL / min. Mark 0.25mL 99m Tc(PVLA)(tricine) 2 Use a 1mL syringe to load the sample, first rinse with 1.25mL eluent, and collect 1mL eluent to obtain a sample that removes impurities and has a recovery rate of >95%. 99m Tc(PVLA)(tricine) 2 solution.
[0080] Identified by chromatography and RP-HPLC, the retention time is 11.9 min, and the radiochemical purity is greater than 99%. HPLC conditions ...
Embodiment 2
[0082] With Bicine as co-ligand, 99m Preparation of Tc(PVLA)(bicine):
[0083] Dissolve 1mg of p(VLA-co-VNI) in 0.5mL phosphate buffer (0.05mol / L pH6.0, containing 20mg co-ligand bicine), and add 2mg / mL SnCl 2 2H 2 O solution 5 μL, shake well. Add fresh at the end 99m TCO 4 - Eluent 0.5mL (37MBq), fully shake, seal and react in boiling water bath for 20min. get 99m Tc(PVLA)(bicine).
[0084] The HiTrap desalting gel column (Sephadex G25) was equilibrated with 25 mL eluent (0.05 mol / L phosphate buffer at pH 7.5), and the flow rate was controlled between 1 and 10 mL / min. Mark 0.25mL 99m Tc(PVLA) (bicine) was loaded with a 1mL syringe, first rinsed with 1.25mL of eluent, and collected 1mL of eluent to obtain a product with the removal of impurities and a recovery rate of >95%. 99m Tc(PVLA) (bicine) solution.
Embodiment 3
[0086] With HEDTA as co-ligand, 99m Preparation of Tc(PVLA)(HEDTA):
[0087] Dissolve 3mg of p(VLA-co-VNI) in 0.5mL phosphate buffer (0.05mol / L pH6.0, containing 50mg co-ligand HEDTA), and add 2mg / mL SnCl 2 2H 2 O solution 20μL, shake well. Add fresh at the end 99m TCO 4 - Eluent 0.5mL (37MBq), fully shake, seal and react in boiling water bath for 20min. get 99m Tc(PVLA)(HEDTA).
[0088] The HiTrap desalting gel column (Sephadex G25) was equilibrated with 25 mL eluent (0.05 mol / L phosphate buffer at pH 7.5), and the flow rate was controlled between 1 and 10 mL / min. Mark 0.25mL 99m Tc(PVLA) (HEDTA) was loaded with a 1mL syringe, first rinsed with 1.25mL of eluent, and collected 1mL of eluent to obtain a product with the removal of impurities and a recovery rate of >95%. 99m Tc(PVLA)(HEDTA) solution.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com