Method of producing n-propyl acetate and allyl acetate
A technology of allyl acetate and n-propyl acetate, which is applied in chemical instruments and methods, preparation of carboxylic acid esters, preparation of hydroxyl compounds, etc., can solve the problem of inability to shift the reaction balance, difficult raw material conversion rate and reaction rate, and large consumption Energy and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
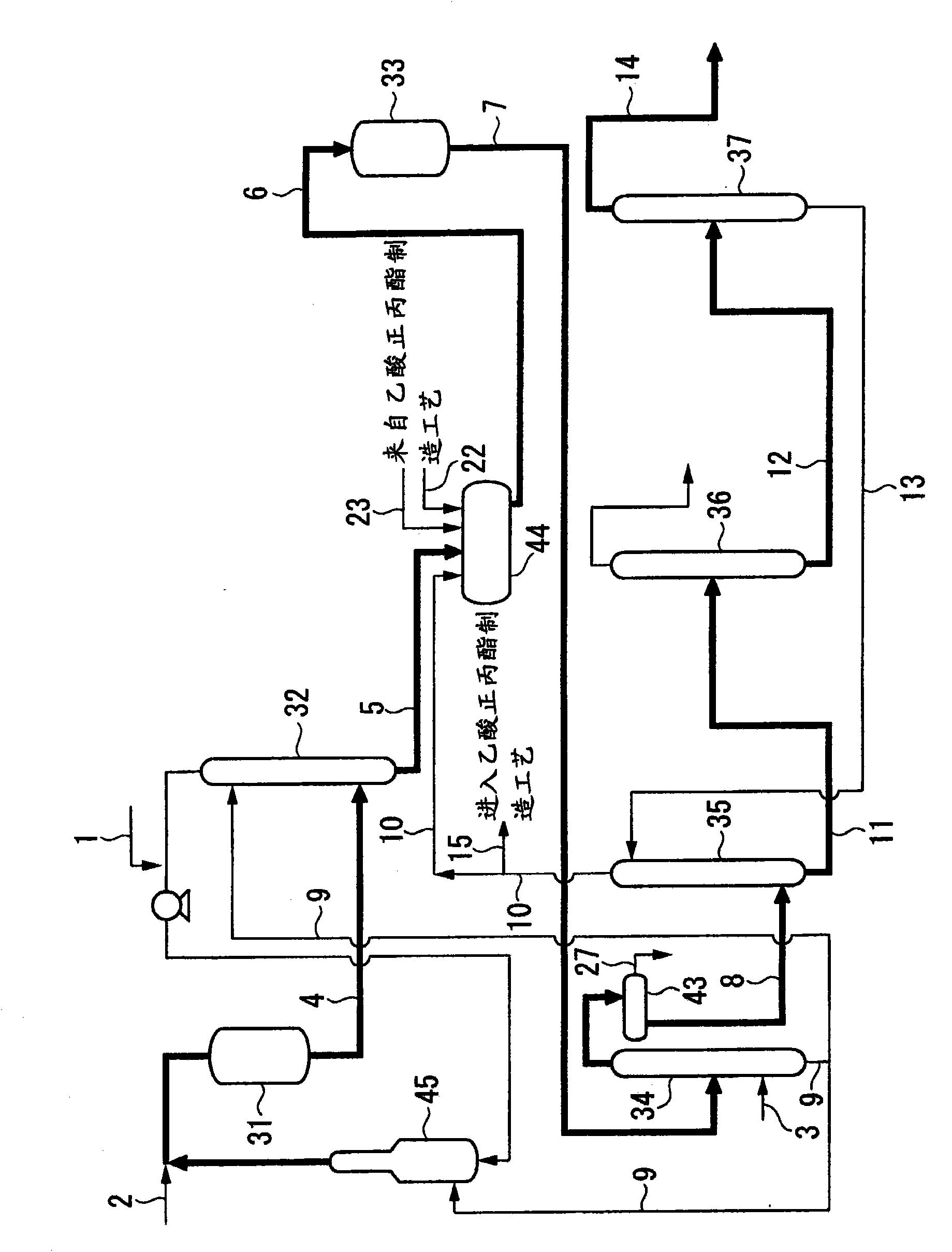
[0195] 13 liters of the extraction column overhead liquid 10 as described above was taken and analyzed in detail. Its composition is shown in Table 1.
[0196] [Table 1]
[0197] components
(weight%)
C3 gas
0.1598
0.0162
0.1001
Isopropyl acetate
0.0110
Isopropanol
0.0278
[0198] components
(weight%)
diallyl ether
0.1034
0.0049
1-propenyl acetate
0.0303
89 to 91
Propyl n-propionate
0.0303
Allyl propionate
0.0880
2-Methylcrotonaldehyde
1.0869
2-Methylbutyraldehyde
0.0000
0.0561
3.0708
0.0795
h 2 o
4.5000
Hazen value
>100
[0199] High-purity allyl acetate was obtained by subjecting the sample liquid to a distillation procedu...
Embodiment 2
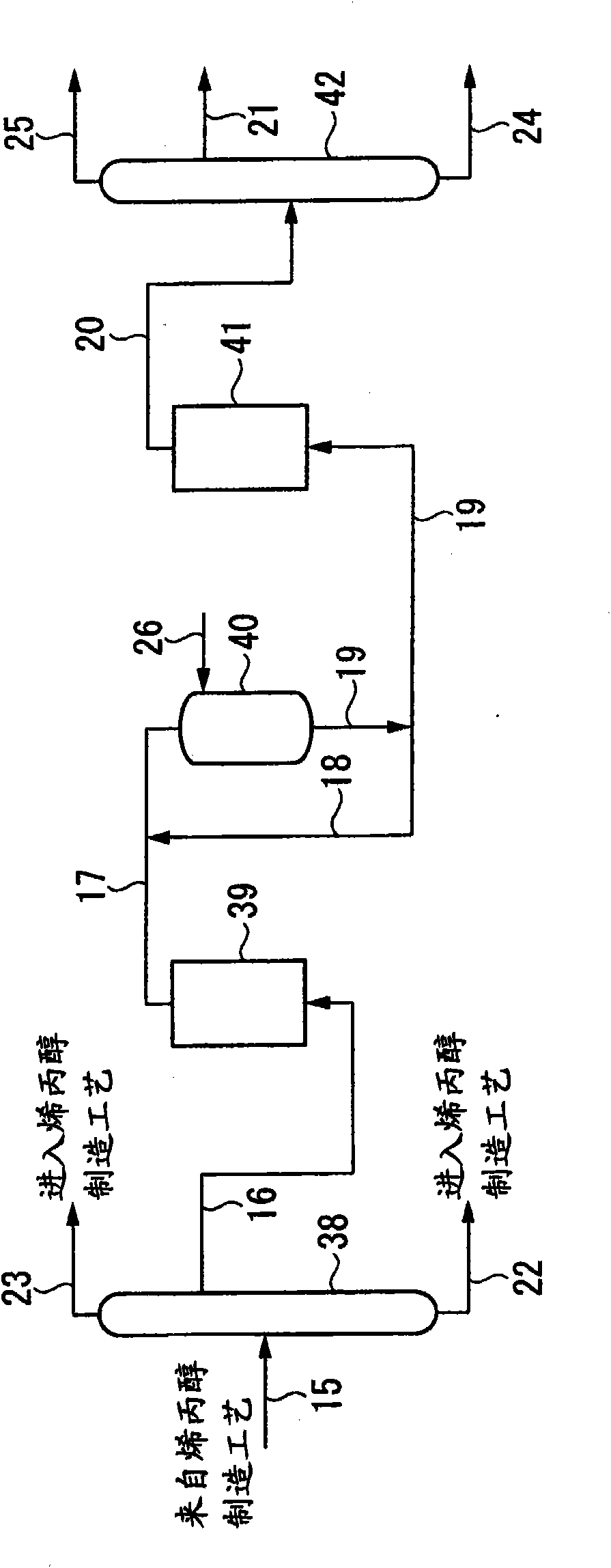
[0262] The same procedure of Example 1 was repeated through the hydrogenation step to obtain compositions as shown in Table 3, and the liquid was then subjected to ozonation according to the method described below.
[0263] Ozone generator: POX-10 / Oxygen (Fuji Electric Co., Ltd.)
[0264] Ozone treatment method: 1.0 liter of the composition shown in Table 3 was charged into a 1 liter graduated cylinder, and ozone-containing gas generated by an ozone generator was diffused from the lower part of the graduated cylinder to allow gas-liquid contact for 2 hours. In addition, the above-mentioned treatment is performed at normal temperature and normal pressure.
[0265] Ozone generator oxygen feed rate: 2.0 L / min
[0266] Ozone generation rate: 2.0 g / h
[0267] The above treatment was repeated five times to treat a total of 4.5 liters of the compositions shown in Table 3 with ozone. The composition of the resulting liquid is shown in Table 5.
[0268] [table 5]
[0269] ...
Embodiment 3
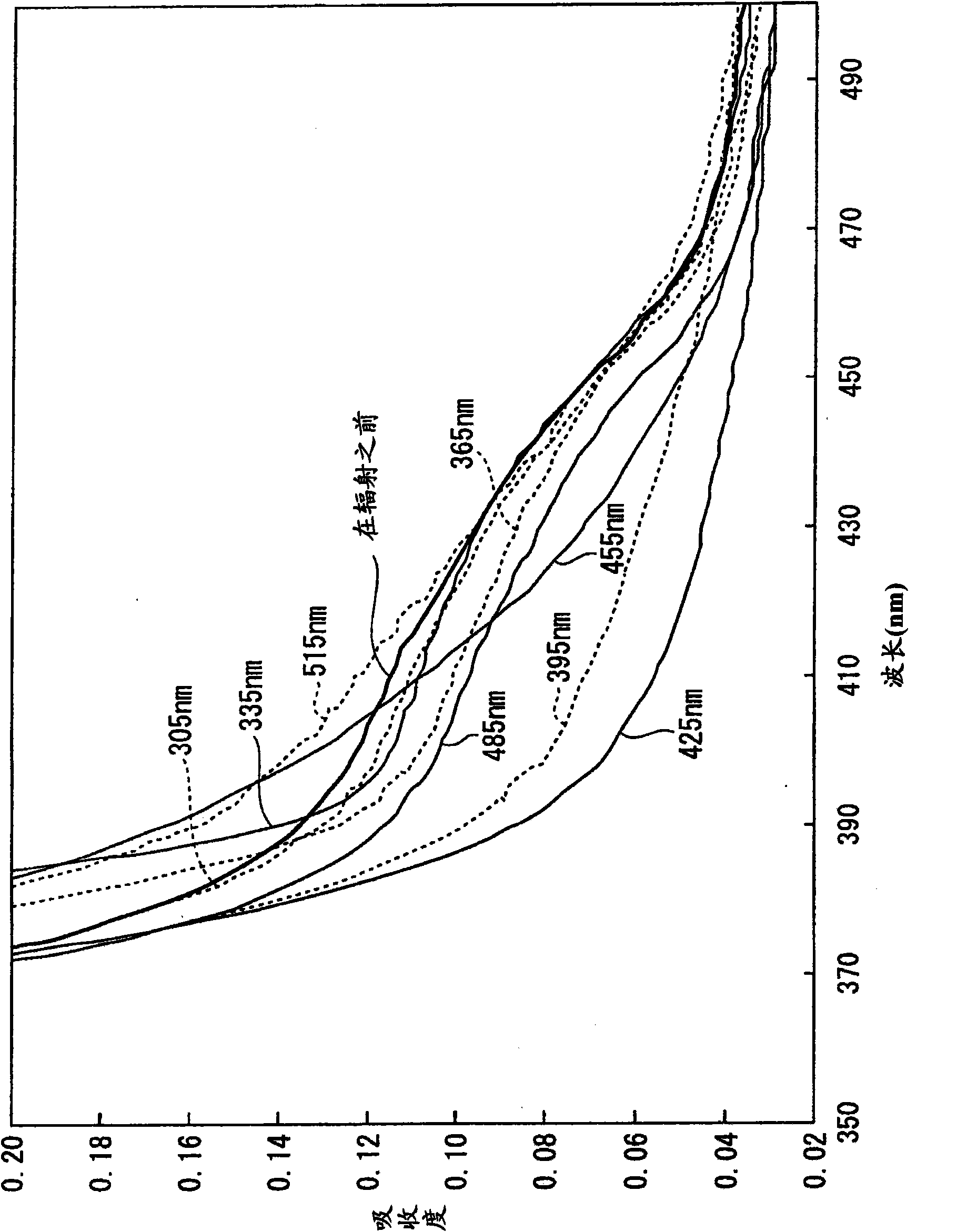
[0296] Approximately 4 liters of the composition of Table 2 described in Example 1 (Fourth Distillation Column Distillate 16) was treated at room temperature between 305 and 515 nm at 4 to 7 mW using the specific wavelength radiation apparatus described below. Irradiate for 25.25 hours at a specified wavelength in wavelength increments of 30 nm, and then measure the optical absorbance of the irradiated liquid at 350 to 500 nm using the optical absorbance measuring device described below. The results are shown in image 3 middle.
[0297] Specific wavelength radiation device: CRM-FD multi-wavelength radiation spectrometer (JASCO Corp.; a device equipped with a 300 W xenon lamp, a focusing parabolic mirror, and a diffraction grating spectrometer, and emits relatively strong monochromatic light. Wavelength accuracy: about 12 nm )
[0298] Optical absorbance measurement device: MPS-2450 spectrophotometer (Shimadzu Corp., double-beam self-recording spectrophotometer, wavelength a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The inside diameter of | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com