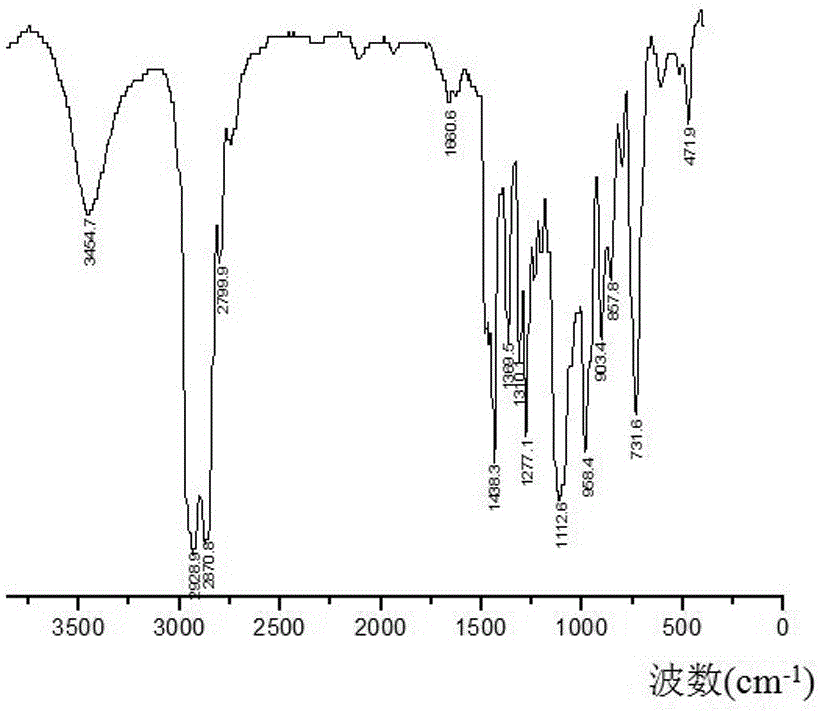
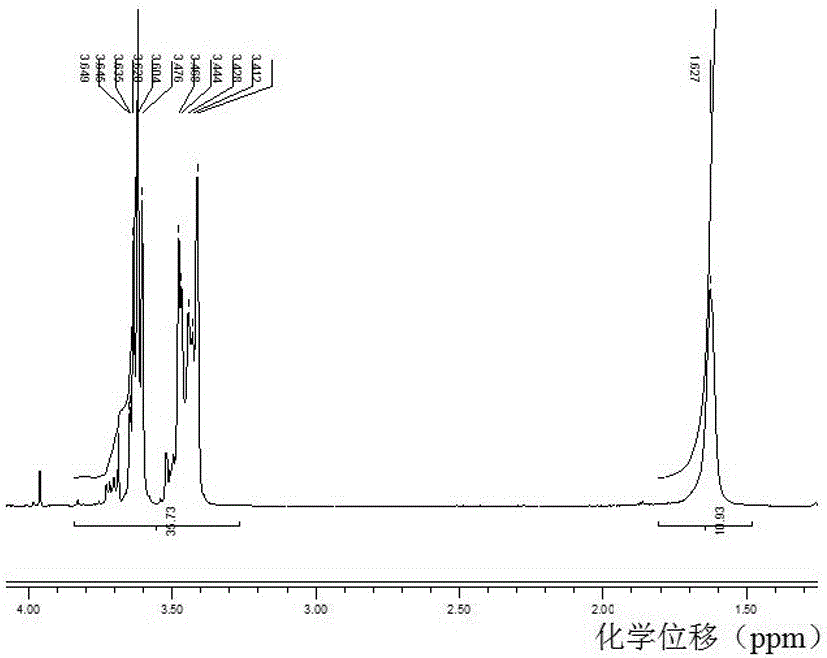
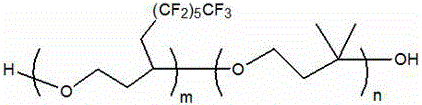
Fluorine-containing polyether glycol and preparation method thereof
A technology of fluorine-containing polyether and diol, which is applied in the field of fluorine-containing polyether diol and its preparation, can solve the problems of difficult preparation of high-molecular-weight polyether and affecting the growth of polyether chains, and achieve a good molecular chain The effects of flexibility, strong hydrophobicity, and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044](1) Synthesis of nonafluorooxetane
[0045] In a 1000mL three-necked flask equipped with a thermometer, ice-water bath and magnetic stirrer protection, add 16.1g of butyl acetate, 26.2g of sodium bicarbonate, 55.8g of sodium dithionite, 227g of N,N-dimethylformamide, 202g of water, 55.3g of perfluorobutyl iodide was added dropwise, reacted at -13°C for 3h, slowly raised to room temperature, and kept for 2h. After the reaction was completed, extraction was performed three times with 180 g of toluene. The extracts were combined and washed three times with water to obtain a solution of 4-nonafluorobutyl-3-iodobutyl acetate, which was directly put into the next step of cyclization reaction without treatment.
[0046] The above-mentioned fluorine-containing alkyl iodide butyl acetate solution was directly added into a 1000mL three-necked flask, and 310g of sodium hydroxide solution with a mass concentration of 20% was added dropwise, and kept at 12°C for 3 hours. After the ...
Embodiment 2
[0054] (1) Synthesis of nonafluorooxetane
[0055] In a 1000mL three-necked flask equipped with a thermometer, ice-water bath and magnetic stirrer protection, add 16.0g butyl acetate, 26.6g sodium bicarbonate, 55.5g sodium dithionite, 231g N,N-dimethylformamide, 208g of water, 55.9g of perfluorobutyl iodide was added dropwise, reacted at -14°C for 3h, slowly raised to room temperature, and kept for 4h. After the reaction was finished, extract with 200 g trifluorotoluene three times. The extracts were combined and washed three times with water to obtain a solution of 4-nonafluorobutyl-3-iodobutyl acetate, which was directly put into the next step of cyclization reaction without treatment.
[0056] The above-mentioned fluorine-containing alkyl iodide butyl acetate solution was directly added into a 1000mL three-necked flask, and 385g of sodium hydroxide solution with a mass concentration of 15% was added dropwise, and kept at 12°C for 3 hours. After the reaction, the layers we...
Embodiment 3
[0062] In a 3000mL three-neck flask equipped with a thermometer, a mechanical stirrer and a constant pressure dropping funnel, add under nitrogen protection, add 565g of dichloromethane and 6.2g of 1,4-butanediol, cool to 1°C in an ice-water bath, and slowly Add 5.5 gBF 3 .Et 2 O, the temperature rises by about 6°C. After 1 hour of reaction, add 292g of 3-(1H,1H-nonafluoropentyl)oxetane and 185g of 3,3-dimethyloxetane dropwise composed of mixed monomers. The control is added for 2 hours. After the addition, the temperature was raised to 30°C, and the reaction was continued for 36h. Stop responding. Adding 102g mass concentration is 2.5% NaHCO 3 Aqueous solution, stirred and reacted for 3 hours, extracted 3 times with 1500g dichloromethane, combined the organic layers, added a desiccant anhydrous magnesium sulfate and dried for 1 hour, filtered to remove the desiccant, and evaporated the filtrate to obtain 412.2g of the product, with a yield of 86.4 %.
[0063] The numbe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com