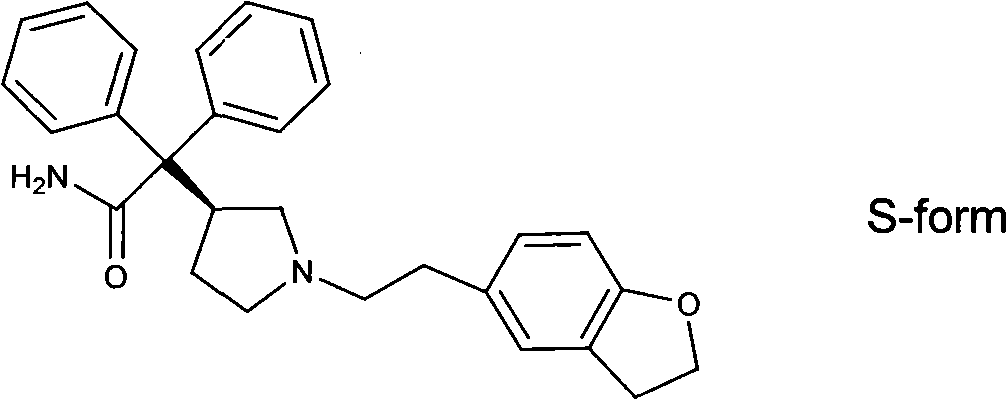
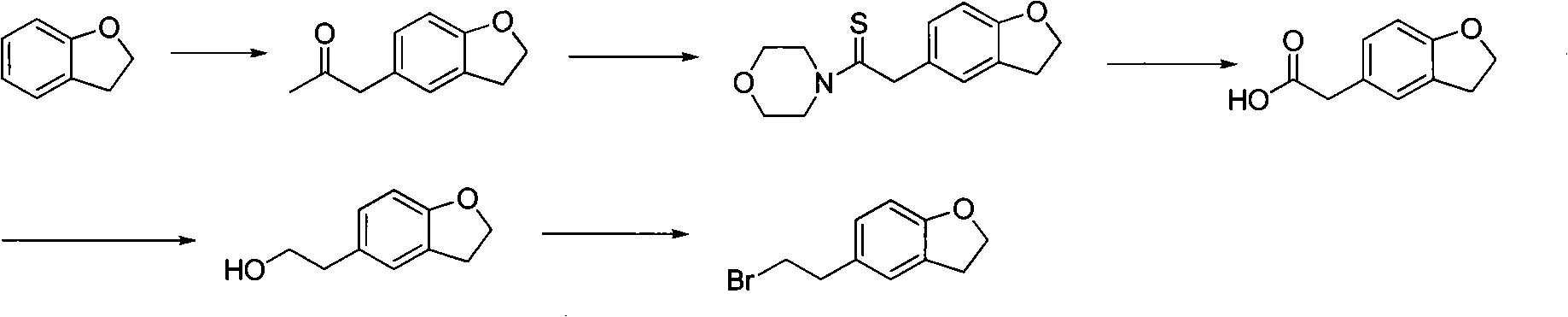
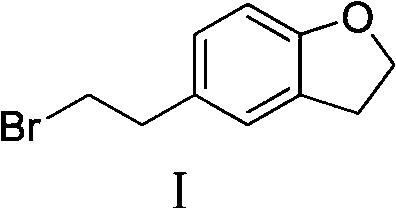
Method for preparing darifenacin intermediate 5-(2-bromoethyl)-2,3-dihydro-1-benzofuran
A technology of benzofuran and dihydrobenzene, which is applied in the field of preparation of darfinazine intermediate 5--2, can solve the problems of unsuitable for industrialization, high cost, low yield, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0025] Example 1. Synthesis of 5-(2-bromoethyl)-2,3-dihydro-1-benzofuran
[0026] 250 ml of tetrahydrofuran, 52.9 g of sodium borohydride, and 71.2 g of 2,3-dihydrobenzofuran-5-acetic acid in 150 ml of THF were added to the reaction flask at 5-10°C. Stir in an ice bath, and when the temperature is lower than 20°C, slowly add 37.3ml of concentrated sulfuric acid dropwise, and stir at room temperature for 2 hours after the drop is completed. After the reaction is completed, add methanol to quench the reaction solution. 300ml, 300ml of 10% NaOH were left to separate and the organic layer was washed with water. Continuously extract the aqueous layer with dichloromethane, combine the organic layers, dry over anhydrous magnesium sulfate, concentrate the organic layer to dryness under reduced pressure, add DMF150ml and stir to dissolve. In an ice bath, slowly add 120 g of phosphorus tribromide dropwise at a temperature of 25 ° C, and react for 1 hour at 45 ~ 50 ° C. After the end, i...
example 2
[0027] Example 2.2, Synthesis of 3-dihydrobenzofuran-5-ethanol
[0028] 250 ml of 1,4-dioxane, 80.7 g of potassium borohydride, and 71.2 g of 2,3-dihydrobenzofuran-5-acetic acid in 150 ml of THF were added to the reaction flask at 5-10°C. Stir in an ice bath, and when the temperature is lower than 20°C, slowly add 127g of iodine in 200ml THF solution dropwise, stir at room temperature for 2 hours after dropping, and react at 60°C for 2 hours, after the reaction is completed, add dilute hydrochloric acid to quench, and the reaction solution is concentrated to dryness , add 100ml of water, 300ml of dichloromethane, 300ml of 20% NaOH and let stand to separate the layers, and the organic layer is washed with water. The aqueous layer was continuously extracted with dichloromethane, the organic layers were combined, dried over anhydrous magnesium sulfate, and the organic layer was concentrated to dryness under reduced pressure to obtain 64.3 g of a white solid. Yield 98%.
example 3
[0029] Example 3.2, Synthesis of 3-dihydrobenzofuran-5-ethanol
[0030] 250 ml of diethylene glycol dimethyl ether, 75.4 g of potassium borohydride, and 71.2 g of 2,3-dihydrobenzofuran-5-acetic acid in 150 ml of THF were added to the reaction flask at 5-10°C. Stir in an ice bath, and when the temperature is lower than 20°C, slowly add 37.3ml of concentrated sulfuric acid dropwise, stir at room temperature for 2 hours after dropping, and react at 5°C for 2 hours. Add 100ml of water, 300ml of dichloromethane, and 300ml of 20% NaOH to separate the layers, and wash the organic layer with water. The aqueous layer was continuously extracted with dichloromethane, the organic layers were combined, dried over anhydrous magnesium sulfate, and the organic layer was concentrated to dryness under reduced pressure to obtain 64.9 g of a white solid. Yield 99%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com