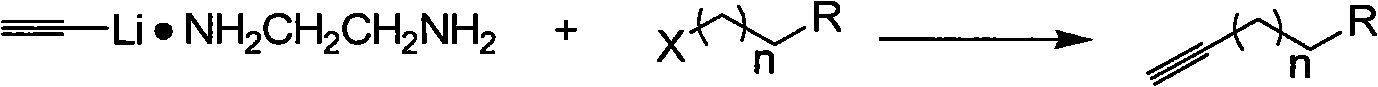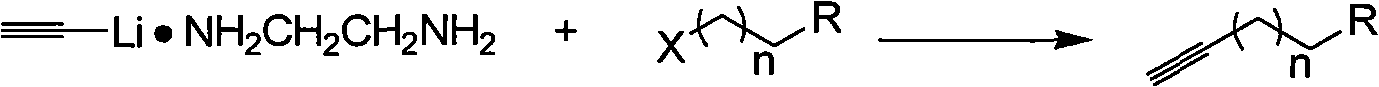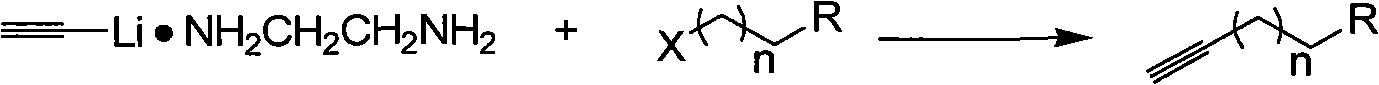Synthesis method of end-group alkyne
A synthesis method and terminal alkyne technology, which is applied in chemical instruments and methods, preparation of hydroxyl compounds, hydrocarbons, etc., can solve the problems of high risk and high equipment requirements, and achieve simple operation, high equipment requirements, and high yield Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0020] A kind of synthetic method of terminal alkynes of the present invention comprises the following steps:
[0021] a. Add the reaction solvent and acetylene lithium ethylenediamine into the flask, cool down to -10-10°C after the solution is clarified, and then add some solvent;
[0022] b, add haloalkane, and control the reaction temperature below 20°C;
[0023] c, 0.5~5h after dripping;
[0024] d, add a large amount of water in the reaction bottle to quench the reaction, separate the liquids, wash with a large amount of water several times, and separate the liquids;
[0025] e, Atmospheric distillation, collecting fractions near the boiling point of the product to obtain the product.
[0026] Its reaction formula is as follows:
[0027]
[0028] In the above reaction formula, X is a halogen, R is an alkyl group, a hydroxyl group, a carboxyl group, and n is 1-8.
[0029] Acetylene lithium ethylenediamine in the above-mentioned preparation method is prepared through...
Embodiment 1
[0035] 1-Hexyne
[0036] (1) Add 50ml DMSO and acetylene lithium ethylenediamine to the reaction flask, and cool down to 10°C after the solution is clarified;
[0037] (2) Add bromobutane dropwise, the molar ratio of bromobutane and acetylene lithium ethylenediamine is 1:1, the heat release is fast, use ice water to cool down, control the reaction temperature to 10-20°C, and add dropwise for 30min;
[0038] (3) After dripping, react at room temperature for 1 hour;
[0039] (4) Add 10% sulfuric acid solution dropwise, adjust pH<7, then add 50ml of water for phase separation;
[0040] (5) Separate the upper oil phase, wash twice with 20 ml of saturated sodium chloride, separate the phases, and dry the organic phase;
[0041] (6) Atmospheric distillation, collecting fractions at about 70-72°C.
Embodiment 2
[0043] 5-Chloro-1-pentyne
[0044] (1) Add 1,3-bromochloropropane and tetrahydrofuran into a 50L kettle, and cool down to -10°C.
[0045] (2) Add the DMSO solution of acetylene lithium ethylenediamine dropwise, so that the molar ratio of 1,3-bromochloropropane to acetylene lithium ethylenediamine is 1:2, the reaction releases heat quickly, stir vigorously, and control the temperature T<-10 ℃.
[0046] (3) The dripping is completed within 2-3 hours, and after the dripping is completed, T<-10°C for 2 hours.
[0047] (4) After the reaction is completed, add water to extract the reaction, stir and let stand to separate layers, wash the upper oil phase with a large amount of water until the organic phase is about 1.5 L, and dry over anhydrous sodium sulfate.
[0048] (5) Atmospheric distillation to obtain the product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com