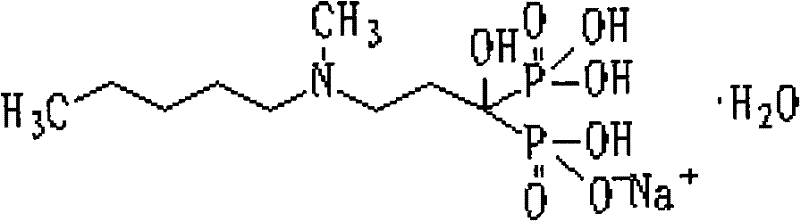
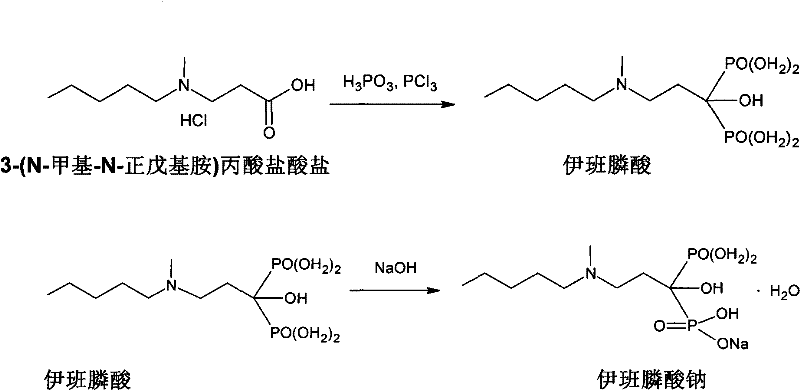
Preparation method of compound sodium ibandronate
A technology for sodium ibandronate and ibandronic acid is applied in the field of synthesis technology of sodium ibandronate, which can solve the problems of large refining loss, difficult to use the process for large-scale production, and inability to uniformly disperse the reaction products, and achieves refining The effect of reducing the number of times and reducing the refining loss
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Raw material name Amount Mole number Mole ratio
[0033] 3-(N-Methyl-N-n-pentylamine) propionate hydrochloride 100 g 0.477 1
[0034] Phosphorus trichloride 79 grams 0.572 1.2
[0035] Phosphorous acid 121 grams 1.479 3.1
[0036] Acetonitrile 400g
[0037] Sodium hydroxide (50%) about 100mL
[0038] water 400mL
[0039] crafting process:
[0040] In a 1L three-neck flask equipped with a reflux device, 100 g of 3-(N-methyl-N-pentylamino) propionate hydrochloride, 121 g of phosphorous acid, and 400 g of acetonitrile were added. Stir, slowly raise the temperature to 70°C, add 79 grams of phosphorus trichloride dropwise, and connect the tail gas absorption device to absorb irritating HCl gas during the dropwise addition. After the phosphorus trichloride was added dropwise, the temperature of the three-neck flask was slowly raised to 95-100°C. It was observed that the reaction product was evenly dispersed in the solvent without lumps. The temperature was kept at 95-10...
Embodiment 2
[0049] Raw material name Amount Mole number Mole ratio
[0050] 3-(N-Methyl-N-n-pentylamine) propionate hydrochloride 100 g 0.477 1
[0051] Phosphorus trichloride 92 grams 0.662 1.39
[0052] Phosphorous acid 121 grams 1.479 3.1
[0053] Acetonitrile 800g
[0054] Sodium hydroxide (50%) about 100mL
[0055] water 400mL
[0056] crafting process:
[0057] In a 2L three-necked flask equipped with a reflux device, 100 g of 3-(N-methyl N-pentylamino) propionate hydrochloride, 121 g of phosphorous acid, and 800 g of acetonitrile were added. Stir, slowly raise the temperature to 70°C, add 92 grams of phosphorus trichloride dropwise, and connect the tail gas absorption device to absorb irritating HCl gas during the dropwise addition. After the phosphorus trichloride was added dropwise, the temperature of the three-neck flask was raised slowly to 95-100°C. It was observed that the reaction product was evenly dispersed in the solvent without lumps. The temperature was kept at 95...
Embodiment 3
[0064] Raw material name Amount Mole number Mole ratio
[0065] 3-(N-Methyl-N-n-pentylamine) propionate hydrochloride 100 g 0.477 1
[0066] Phosphorus trichloride 83 grams 0.596 1.25
[0067] Phosphorous acid 121 grams 1.479 3.1
[0068] Acetonitrile 600g
[0069] Sodium hydroxide (50%) about 100mL
[0070] water 400mL
[0071] crafting process:
[0072] In a 2L three-neck flask equipped with a reflux device, 100 g of 3-(N-methyl-N-pentylamino) propionate hydrochloride, 121 g of phosphorous acid, and 600 g of acetonitrile were added. Stir, slowly raise the temperature to 70°C, add 83 grams of phosphorus trichloride dropwise, and connect the tail gas absorption device to absorb irritating HCl gas during the dropwise addition. After the phosphorus trichloride was added dropwise, the temperature of the three-neck flask was raised slowly to 95-100°C. It was observed that the reaction product was evenly dispersed in the solvent without lumps. The temperature was kept at 95-1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com