Synthesis method of high-purity fluorine-doped lithium iron phosphate anode material
A technology of electrode materials and mixtures, which is applied in the field of controllable synthesis of high-purity fluorine-doped lithium iron phosphate cathode materials, can solve the problems of low product purity and expensive raw materials, and achieve simple synthesis process, low price and high yield high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
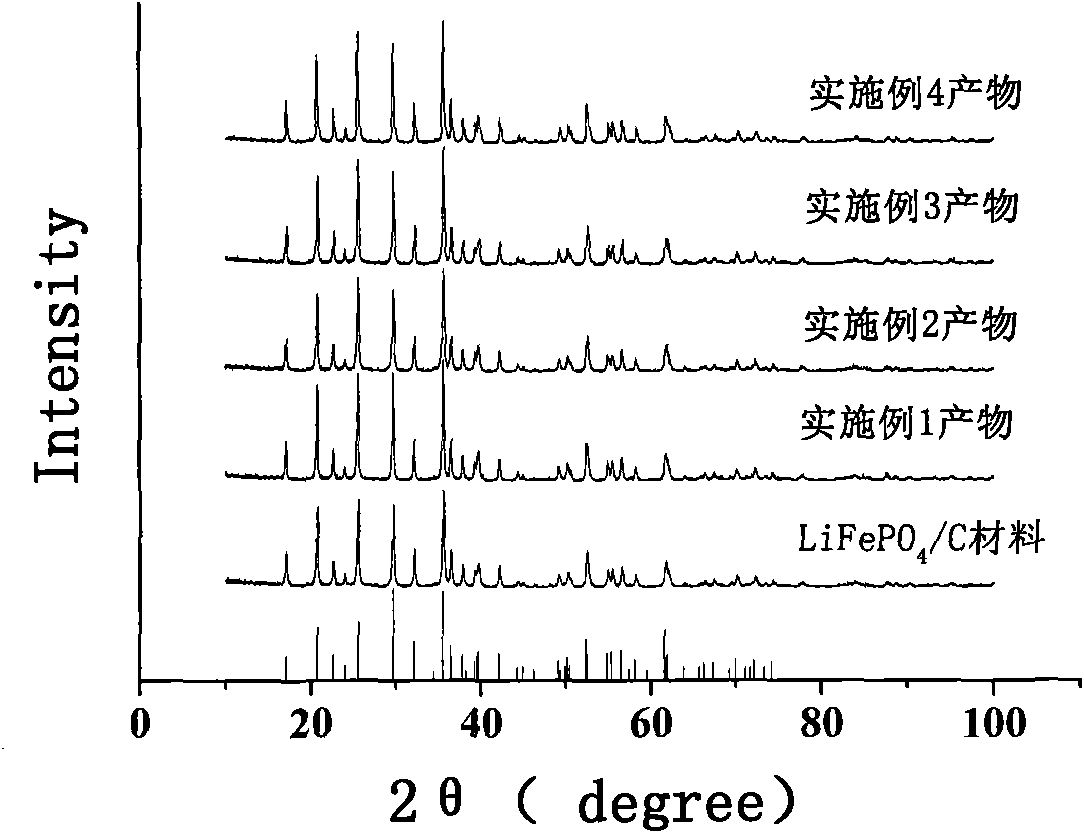
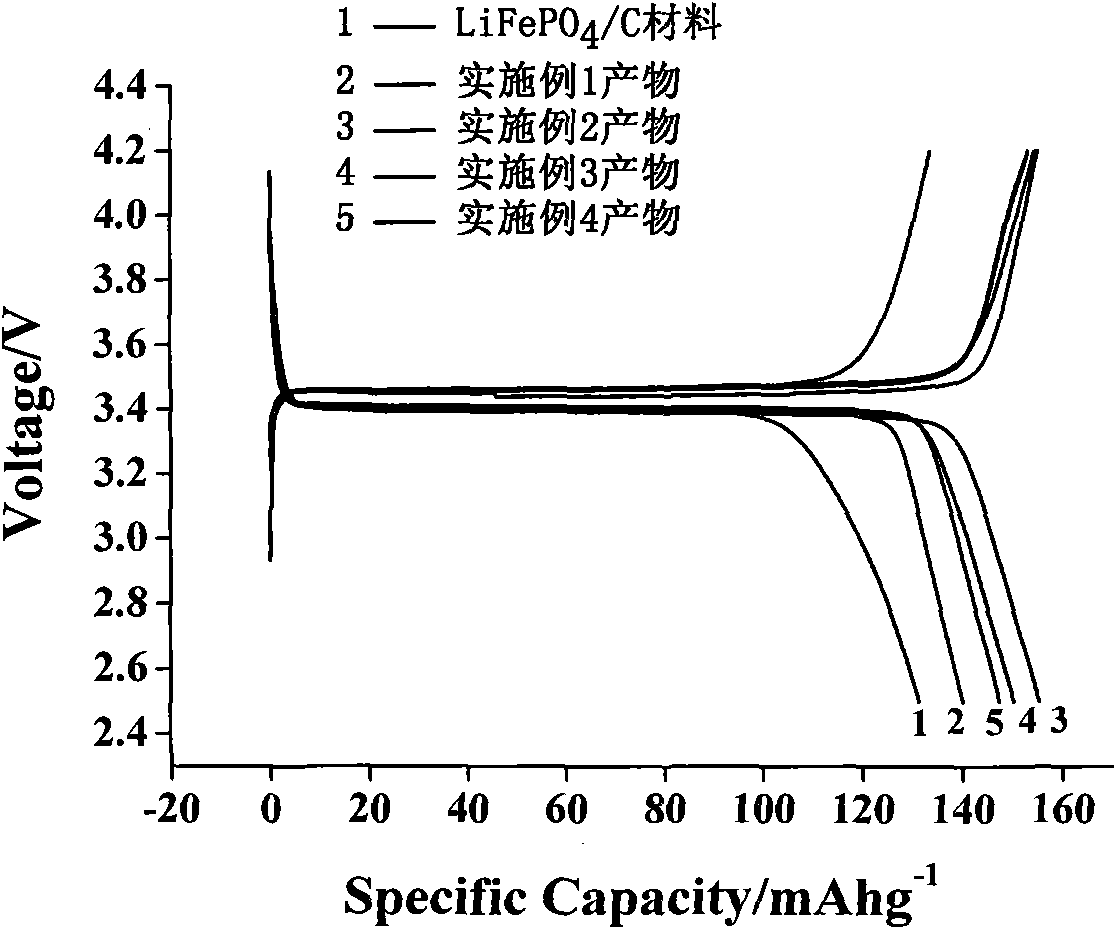
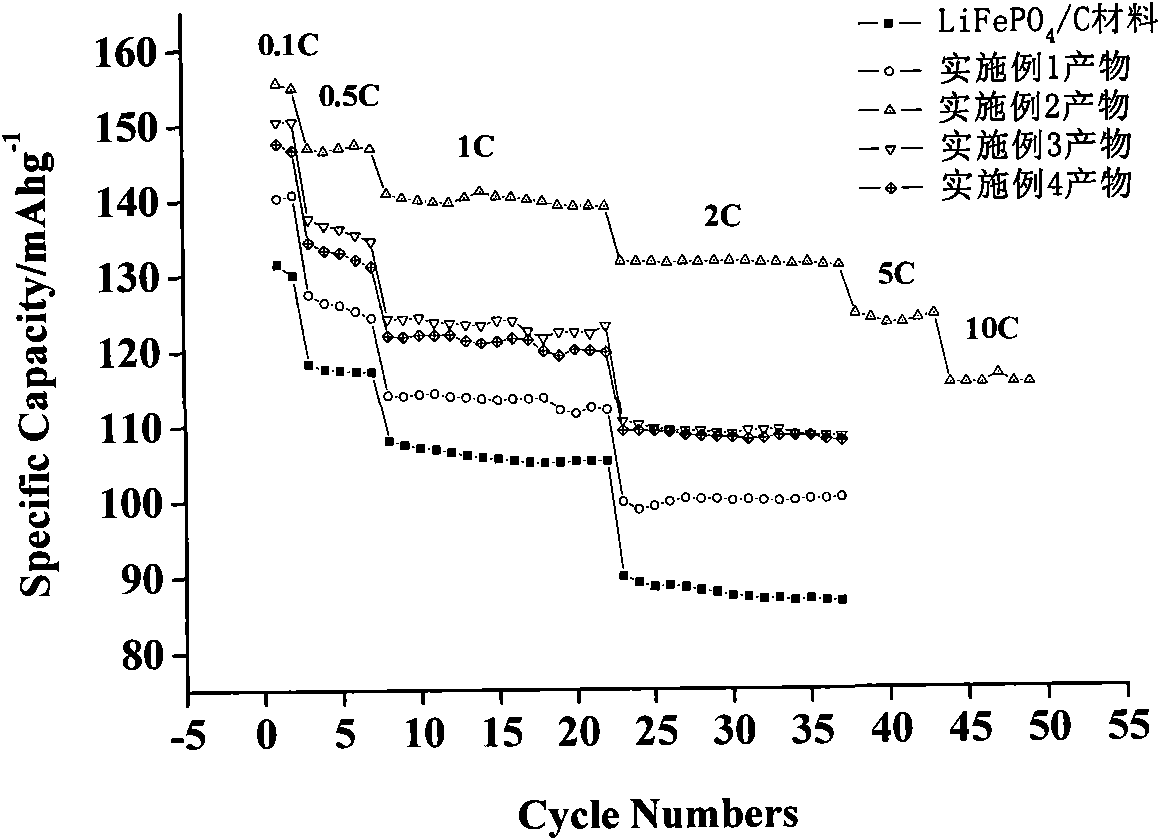
Image
Examples
Embodiment 1
[0024] LiFe(PO 4 ) 0.996 f 0.01 Preparation of / C material
[0025] Step 1: Put the analytically pure reagent LiOH·H 2 O, FeC 2 o 4 2H 2 O, NH 4 h 2 PO 4 and LiF, mixed according to the stoichiometric ratio of 1.01:1:0.996:0.01, adding 10% sucrose of the final product mass, using absolute ethanol as the medium, milling in a ball mill at a speed of 450r / min for 11h, and drying for 15h to obtain the mixture .
[0026] Step 2: Put the mixture prepared in Step 1 into the porcelain ark, and raise the temperature to 350°C at 10°C / min in a tube furnace under an argon atmosphere, keep it warm for 7 hours, and then raise it to 650°C at 10°C / min ℃, sintered for 15 hours, and the product was taken out when it was cooled to room temperature, and the final product was obtained.
Embodiment 2
[0028] LiFe(PO 4 ) 0.993 f 0.02 Preparation of / C material
[0029] Step 1: Put the analytically pure reagent LiOH·H 2 O, FeC 2 o 4 2H 2 O, NH 4 h 2 PO 4 and LiF, mixed according to the stoichiometric ratio of 1:1:0.993:0.02, adding 10% sucrose of the final product mass, using absolute ethanol as the medium, ball milling at a speed of 450r / min in a ball mill for 11h, and drying for 15h to obtain the mixture .
[0030] Step 2: Put the mixture prepared in Step 1 into the porcelain ark, and raise the temperature to 300°C at 10°C / min in a tube furnace under an argon atmosphere, keep it warm for 5 hours, and then raise it to 650°C at 10°C / min ℃, sintered for 10h, and the product was taken out when it cooled to room temperature, and the final product was obtained.
Embodiment 3
[0032] LiFe(PO 4 ) 0.99 f 0.03 Preparation of / C material
[0033] Step 1: Put the analytically pure reagent LiOH·H 2 O, FeC 2 o 4 2H 2 O, NH 4 h 2 PO 4 and LiF, mixed according to the stoichiometric ratio of 0.99:1:0.99:0.03, adding 10% sucrose of the final product mass, using absolute ethanol as the medium, milling in a ball mill at a speed of 450r / min for 11h, and drying for 15h to obtain the mixture .
[0034]Step 2: Put the mixture prepared in Step 1 into the porcelain ark, and raise the temperature to 350°C at 15°C / min in a tube furnace under an argon atmosphere, keep it warm for 6 hours, and then raise it to 650°C at 15°C / min ℃, sintered for 12 hours, and the product was taken out when it was cooled to room temperature, and the final product was obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com