Preparation method for tetra-alkyl ammonium hydroxide and application
A technology of tetraalkylammonium halide and ammonium hydroxide, which is applied in the preparation of amino compounds, organic compounds, and hydrogen atoms replaced by amino groups. It can solve the problems of high equipment requirements, cumbersome operation, and high energy consumption. Low cost, convenient operation and low energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
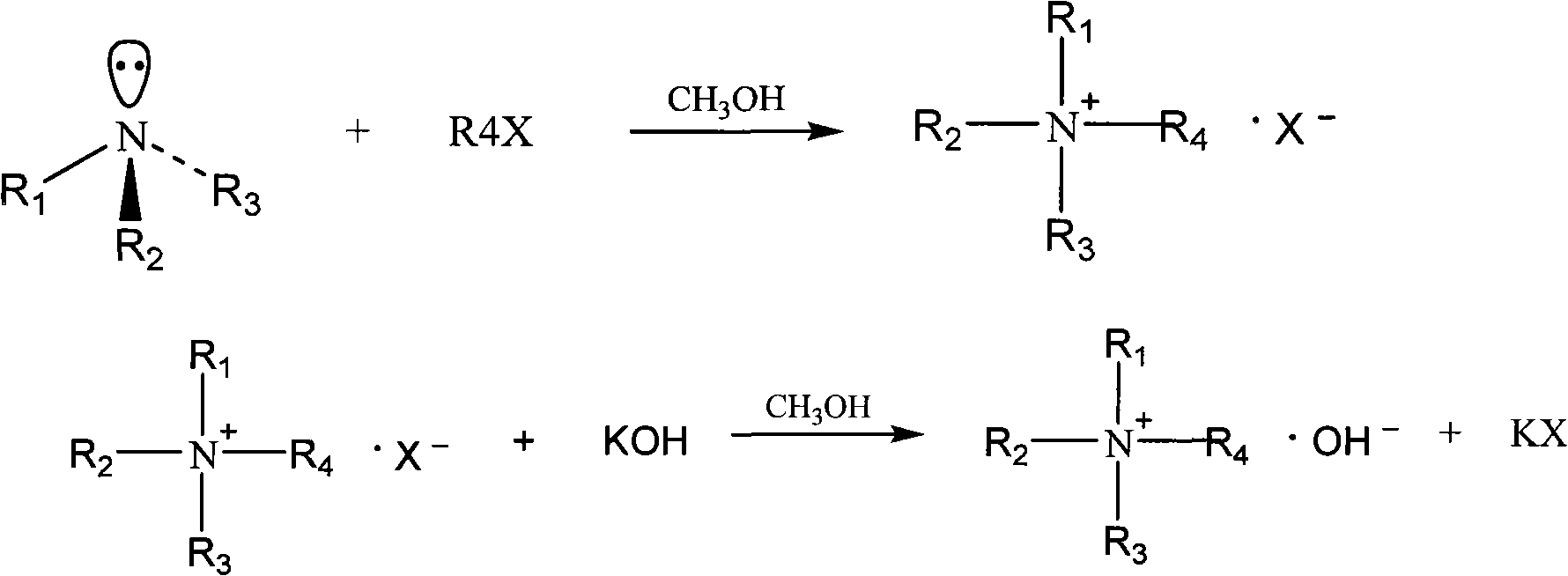
Method used
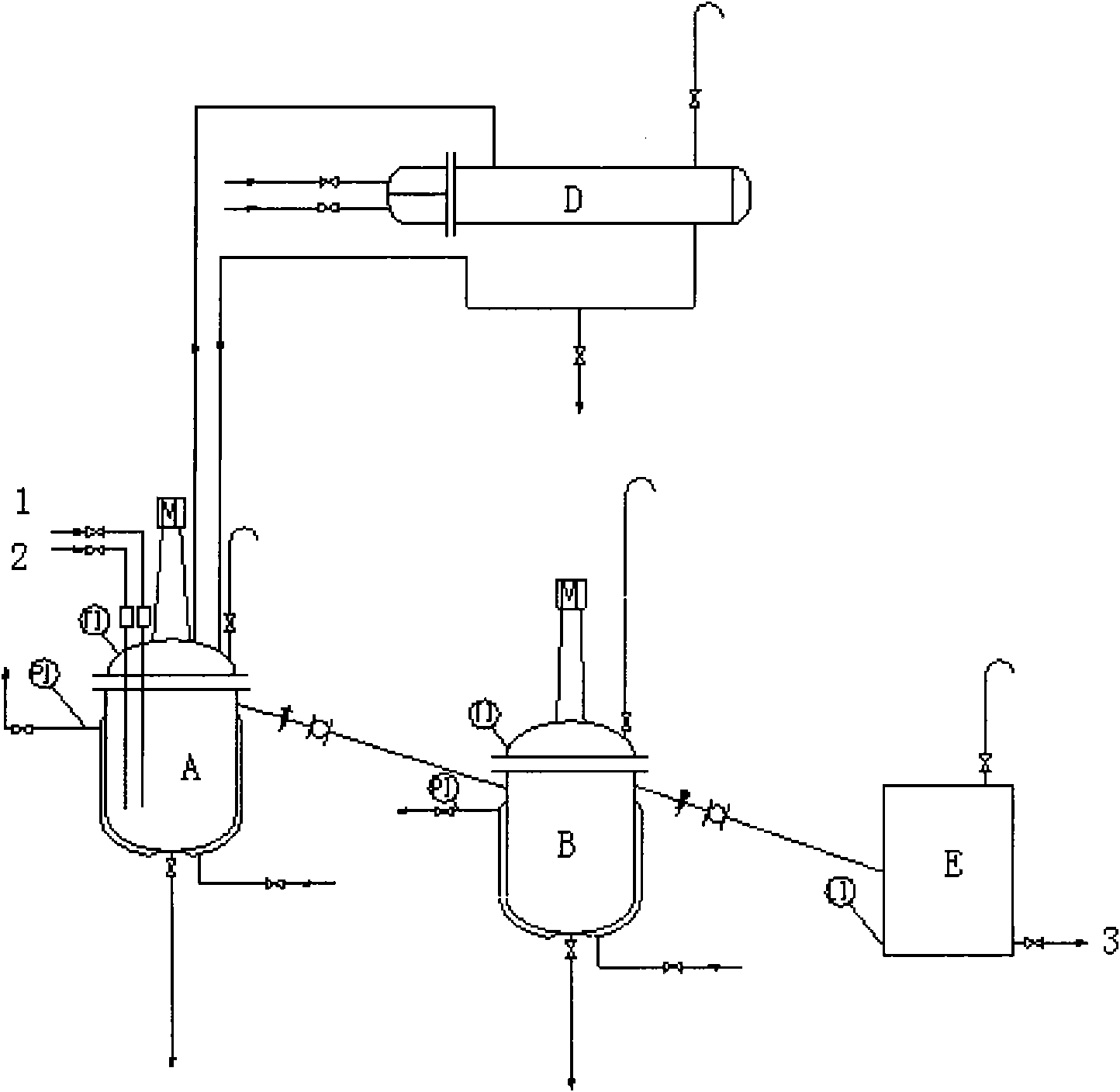
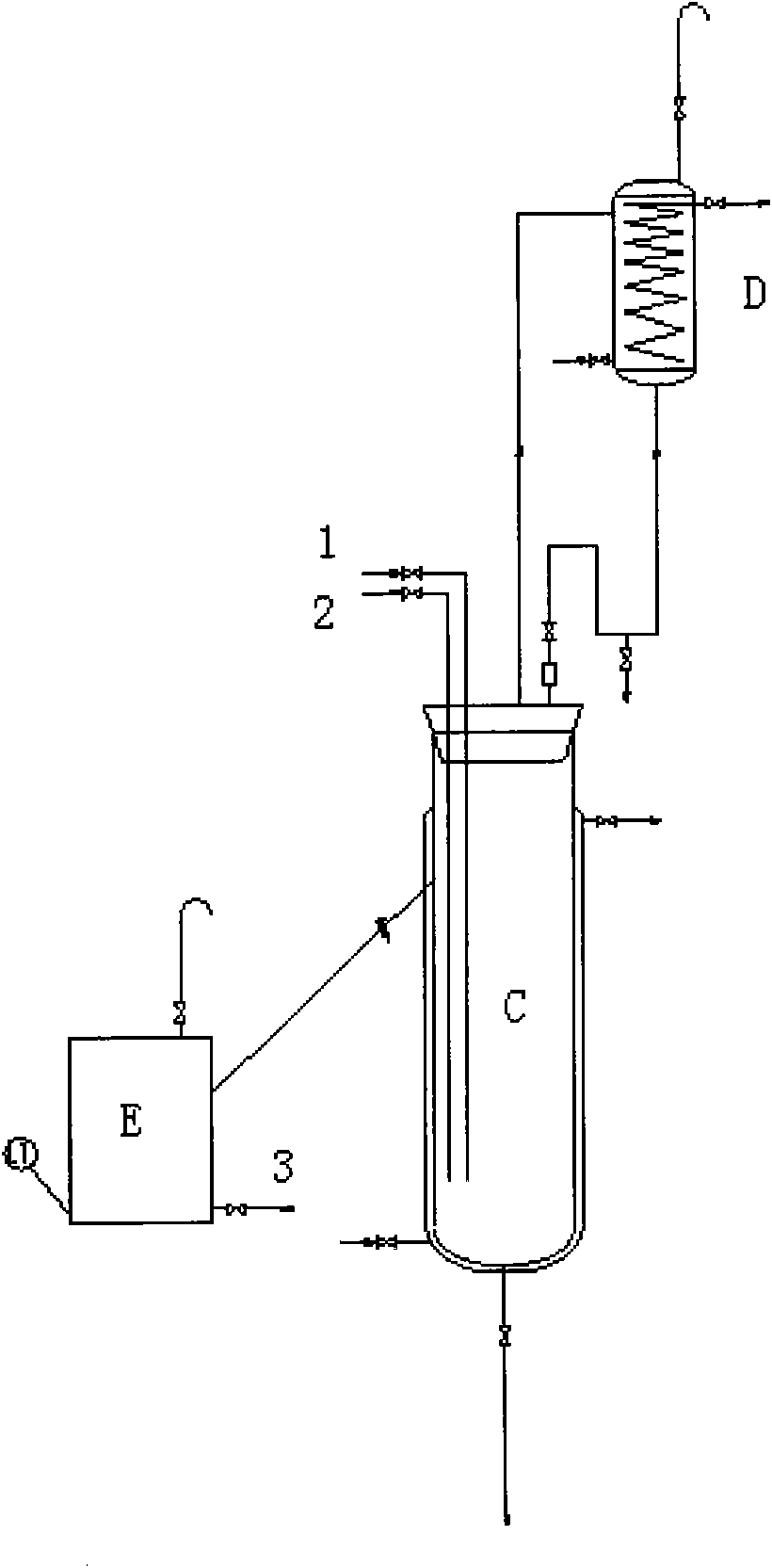
Image
Examples
Embodiment 1
[0038] Operation step 1:
[0039] Add 529.56g of trimethylamine methanol solution containing 26.65% of trimethylamine into a 1L reactor, start to feed in methyl chloride gas under stirring, keep the temperature of the reaction solution at 30-35°C, ventilate for about 4 hours, stop ventilating, and the weight of the reaction solution increases About 143.1 g, pH=7. After the reaction, the reaction solution was heated and refluxed for 0.5h, the methyl chloride was absorbed by methanol, and the temperature was lowered to obtain a tetramethylammonium chloride methanol solution, weighing 647.78g. The content of tetramethylammonium chloride (TMACl) was determined to be 40.26%, and the yield was ≥ 99.5%.
[0040] Operation step 2:
[0041] Add 91.88g KOH (1.575mol) to 1L reactor and dissolve in 230g anhydrous methanol, add 391.65g (1.44mol) of the above-mentioned TMACl methanol solution under stirring, and keep warm at 60-70°C for 2h. After the heat preservation is completed, cool ...
Embodiment 2
[0043] Operation step 1:
[0044] Add 588g of trimethylamine methanol solution containing 15% trimethylamine into a 1L reactor, and start to feed in methyl chloride gas under stirring, keep the temperature of the reaction liquid at 20-25°C, ventilate for about 5 hours, stop ventilating, and the weight of the reaction liquid increases by about 79.24g, pH=7. After the reaction, the reaction solution was heated and refluxed for 0.5h, the methyl chloride was absorbed with methanol, and the temperature was lowered to obtain tetramethylammonium chloride methanol solution, weighing 662.1g, and the measured tetramethylammonium chloride (TMACl) content was 24.48%, and the yield≥ 99%.
[0045] Operation step 2:
[0046] Add 68.97g KOH (1.17mol) in 1L reactor and dissolve in 240g anhydrous methanol, add 523.34g (1.17mol) of the above TMACl methanol solution under stirring, and keep warm at 30-35°C for 3h. After the heat preservation is completed, cool down to 20°C with water, desalt t...
Embodiment 3~4
[0048] Change raw material methyl bromide to replace methyl chloride, other conditions are identical with embodiment 1, 2, the results are shown in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com