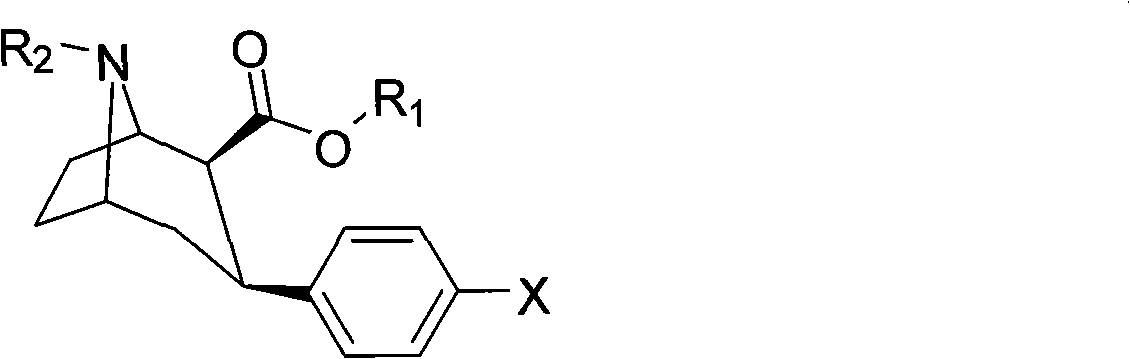
Dopamine transporter imaging medicine and preparation method thereof
A transporter and dopamine technology, applied in the field of dopamine transporter imaging drugs and its preparation, can solve the problems of poor specific selectivity, low ratio of drug target to non-target, and poor stability in vivo
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
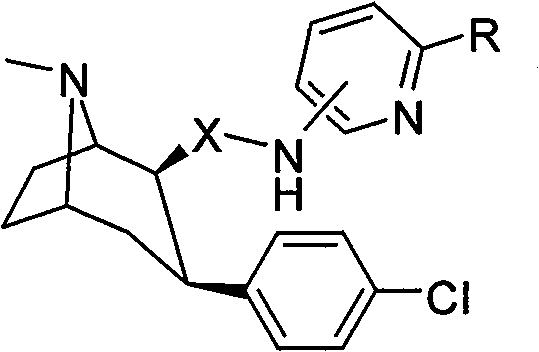
[0032] Synthesis of 2β-(N-2-bromopyridine-6-carboxamide)-3βchlorobenzene A-1
[0033] (1) The structure of the compound
[0034]
[0035] (2) Synthesis method
[0036] Add 280mg (1mM) 2β-carboxy-3βchlorophentropine and 5ml of anhydrous dichloromethane in a single-necked round-bottomed flask, add dropwise an excess of 1.5ml of oxalyl chloride in dichloromethane solution, and react at room temperature After 1.5 hours, the solvent and unreacted oxalyl chloride were pumped off with a water pump, and then dried with an oil pump to obtain a foamy solid. Then add 5 ml of dichloromethane to dissolve the obtained solid, and add 172 mg (1 mM) of 6-amino-2-bromopyridine and 0.14 ml (1 mM) of triethylamine under nitrogen protection using an ice-salt bath. After reacting at room temperature for 12 hours, stop the reaction, wash the reaction solution repeatedly with deionized water, and back-extract with dichloromethane, combine the dichloromethane layers, dry over anhydrous sodium sul...
Embodiment 2
[0042] Synthesis of 2β-(N-2-bromopyridine-6-methylamine)-3βchlorobenzene B-1
[0043] (1) The structure of the compound
[0044]
[0045] (2) Synthesis method
[0046] Dissolve 0.5g of A-1 in 4ml of THF, add dropwise 3ml of 1M borane THF solution under nitrogen protection, heat to reflux, react for 12 hours, then stop the reaction, remove the solvent under reduced pressure, add 3mL of 1N hydrochloric acid to the residue , boiled for 30min, cooled to room temperature, neutralized with 5mL ammonia water, the obtained precipitate was extracted with dichloromethane, dried over anhydrous sodium sulfate, purified by silica gel chromatography, the solvent ratio was ethyl acetate: n-hexane: triethylamine=6:4 : 0.3, a yellow foamy solid was obtained with a yield of 70%.
[0047] (3) Confirmation of B-1
[0048] h 1 -NMR (CDCl 3, 200MHz): δ (ppm) 1.5-1.8 (m, 3H), 1.8-2.0 (m, 2H), 2.1-2.5 (m, 2H), 2.4-2.5 (s, 3H), 2.8-2.9 (dt, 1H), 3.1-3.3(dt, 2H), 3.3-3.4(t, 2H), 5.9-6.1(d, 2H)...
Embodiment 3
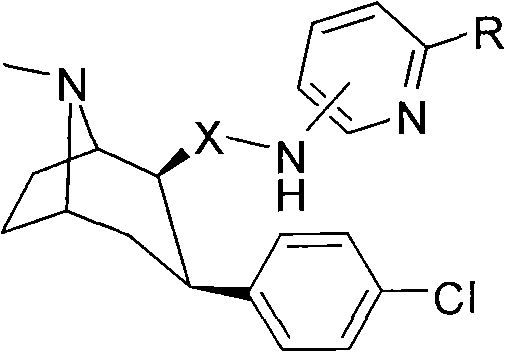
[0052] Synthesis of 2β-(N-2-bromopyridine-5-carboxamide)-3βchlorobenzene A-2
[0053] (1) The structure of the compound
[0054]
[0055] (2) Synthesis method
[0056] Add 280mg (1mM) 2β-carboxy-3βchlorophentropine and 5ml of anhydrous dichloromethane in a single-necked round-bottomed flask, add dropwise an excess of 1.5ml of oxalyl chloride in dichloromethane solution, and react at room temperature After 1.5 hours, the solvent and unreacted oxalyl chloride were pumped off with a water pump, and then dried with an oil pump to obtain a foamy solid. Then add 5 ml of dichloromethane to dissolve the obtained solid, and add 172 mg (1 mM) of 5-amino-2-bromopyridine and 0.14 ml (1 mM) of triethylamine under nitrogen protection using an ice-salt bath. After reacting at room temperature for 12 hours, stop the reaction, wash the reaction solution repeatedly with deionized water, and back-extract with dichloromethane, combine the dichloromethane layers, dry over anhydrous sodium sul...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com