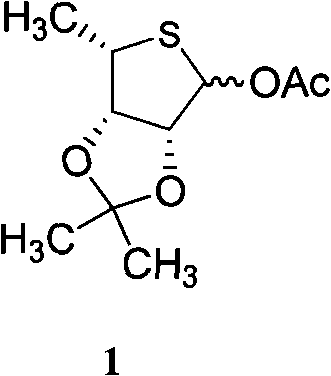
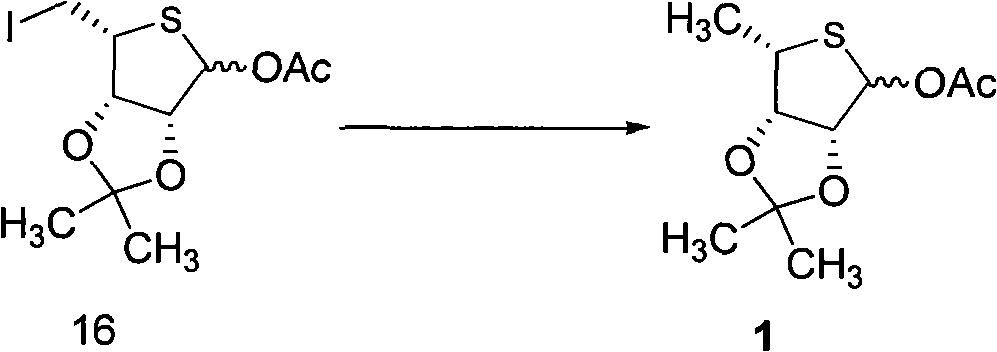
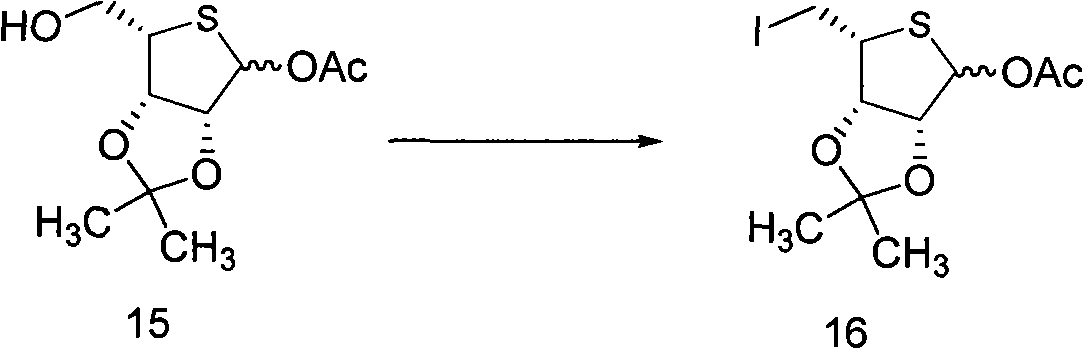
Intermediate compounds for thiophane nucleoside analogues and preparation method thereof
A technology of thiophene nucleosides and thiophene nucleosides, which is applied in the fields of silicon organic compounds and organic chemistry, and can solve problems such as high cost, harsh conditions, and long reaction routes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1: (2S, 3S, 4R)-2-tert-butyldimethylsiloxymethyl-3,4-O-isopropylidene-tetrahydrothiophene (compound 12)
[0034] Compound 11 (32.1 g, 0.07 mol) was dissolved in 700 mL of dimethyl sulfoxide, sodium sulfide hydrate (20 g, 0.08 mol) was added at room temperature, mechanically stirred at 100°C, and monitored by TLC until the disappearance of the raw material. After cooling down to room temperature, the reaction system was poured into ice water, stirred for 5 minutes, and extracted with ether five times (200 mL / time). The organic layers were combined, washed with water 3 times (200 mL / time), dried over anhydrous magnesium sulfate and concentrated to obtain a crude yellow oil. The crude product was purified by column chromatography (petroleum ether / ethyl acetate 20 / 1) to obtain 13.8 g of a yellow oil with a yield of 65%.
[0035] [α] D 20 =194°C=1.0, Methanol.
[0036] MS m / z: 305 (M + +1). 1 HNMR (CDCl 3 )δ (ppm): 0.89 (s, 9H); 0.08 (s, 6H); 1.29 (s, 3H); 1.4...
Embodiment 2
[0037] Example 2: (2S, 3S, 4R)-1-oxo-2-tert-butyldimethylsiloxymethyl-3,4-O-isopropylidene-tetrahydrothiophene (compound 13)
[0038] Compound 12 (7.4 g, 24.3 mmol) was dissolved in 150 mL of dichloromethane. At -78°C, 0.49mol / L m-chloroperoxybenzoic acid in dichloromethane (50mL, 24.5mmol) was added dropwise, reacted for 1 hour, then warmed up to room temperature, added saturated aqueous sodium bicarbonate, and stirred for 5 minutes. The layers were separated, the aqueous layer was extracted three times with dichloromethane (30 mL / time), the organic layers were combined, and the organic layer was washed with saturated brine. Dry over anhydrous magnesium sulfate and concentrate to give a white solid. After purification by column chromatography (petroleum ether / ethyl acetate 3 / 1), 3.97 g of a white solid was obtained, with a yield of 51%.
[0039] [α] D 20 =153°C=1.0, Methanol.
[0040] MS m / z: 343 (M + +23). 1 HNMR (CDCl 3 )δ (ppm): 0.93 (s, 9H); 0.12 (S, 6H); 1.30 (S,...
Embodiment 3
[0041] Example 3: (2S, 3S, 4R)-1-oxo-2-tert-butyldimethylsiloxymethyl-3,4-O-isopropylidene-tetrahydrothiophene (compound 13)
[0042] Compound 12 (7.4 g, 24.3 mmol) was dissolved in 150 mL of dichloromethane. At -10°C, 0.49 mol / L m-chloroperbenzoic acid-added dichloromethane solution (50 mL, 24.5 mmol) was added dropwise. After reacting for 1 hour, it was raised to room temperature, and saturated aqueous sodium bicarbonate solution was added, and stirred for 5 minutes. The layers were separated, and the aqueous layer was extracted three times with dichloromethane (30 mL / time). The organic layers were combined and washed with saturated brine. Dry over anhydrous magnesium sulfate, filter and concentrate to obtain a white solid. Purified by column chromatography (petroleum ether / ethyl acetate 3 / 1) to obtain a white solid with a yield of 48%.
[0043] [α] D 20 =153°C=1.0, Methanol.
[0044] MS m / z: 343 (M + +23). 1 HNMR (CDCl 3 )δ (ppm): 0.93 (s, 9H); 0.12 (S, 6H); 1.30 (S, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com