Klebsiella pneumoniae (Mannose-sensitive hemagglutination) pilus strain
A Klebsiella, mannose technology, applied in bacteria, antibacterial drugs, allergic diseases, etc., can solve problems such as no medical application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Example 1. Source and identification of Klebsiella pneumoniae mannose-sensitive hemagglutination strains
[0059] The inventors established a mannose-sensitive hemagglutination fimbriae strain of Pseudomonas aeruginosa by comparing 6 strains of Klebsiella pneumoniae isolated from clinical specimens, reference (Muxia, Pseudomonas aeruginosa, Acta Microbiology, 26(2): 176-179 , 1986), screened and bred to obtain a Klebsiella pneumoniae mannose-sensitive hemagglutination strain.
[0060] The main identification features are as follows:
[0061] Fermentation of lactose on differential medium to express colored colonies;
[0062] On solid medium, it is a gray-white sticky peptone-like colony;
[0063] After several days in the broth, the liquid is noticeably viscous;
[0064] Gram negative.
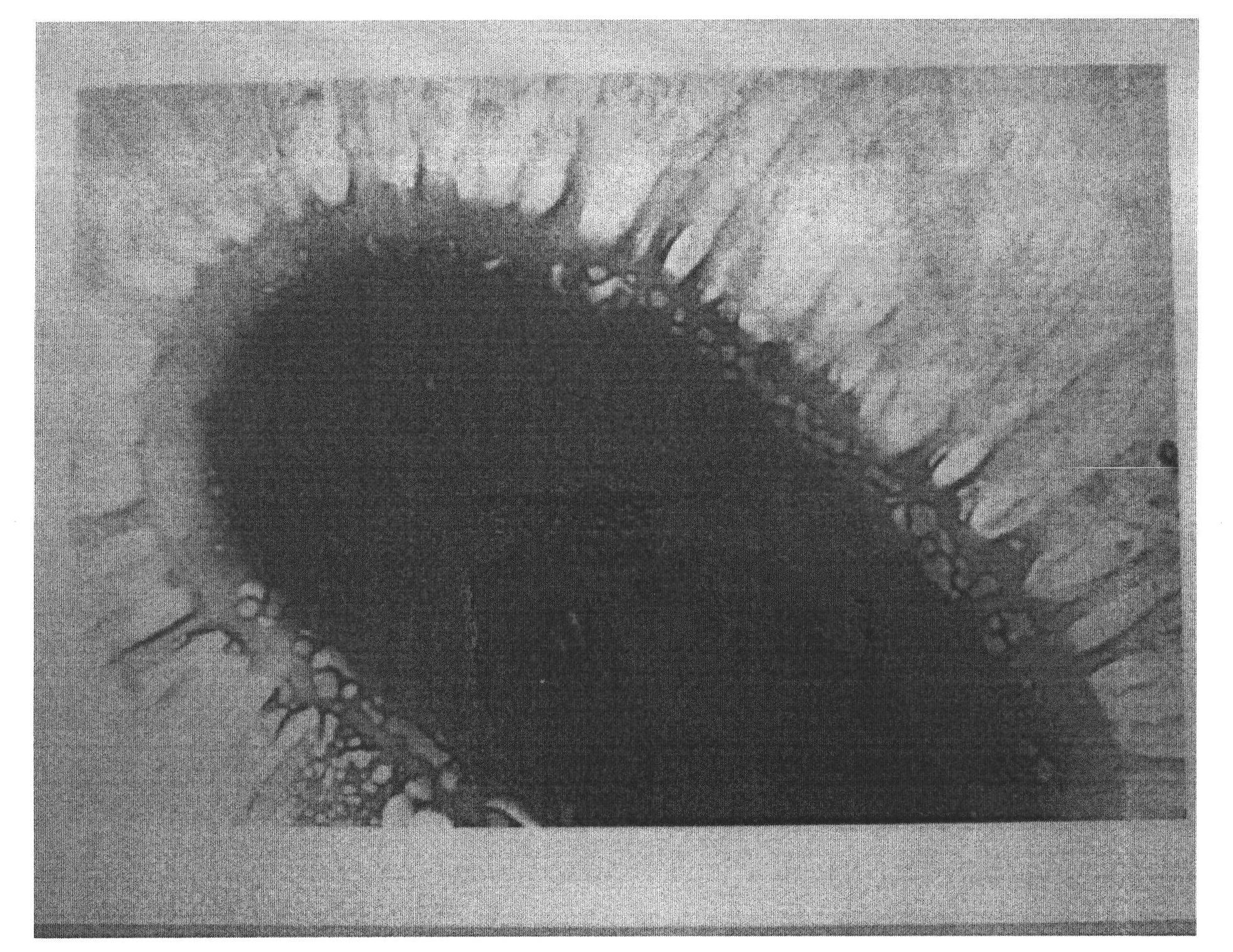
[0065] Electron micrographs were performed on this strain, such as figure 1 shown, showing the presence of periphytic fimbriae. Mannose-sensitive hemagglutination and hemagglutina...
Embodiment 2
[0066] Example 2. Strain culture and vaccine preparation
[0067] The Klebsiella pneumoniae mannose-sensitive hemagglutination fimbriae strain of the present invention is cultured on a bacterial plate at 37° C. for 18 hours, the culture is collected, sterilized with a formaldehyde solution, washed repeatedly to prepare a dead bacterin, diluted with Bacterial saline was added with 1% benzyl alcohol as a preservative to prepare a bacterin.
Embodiment 3
[0068] Example 3. Animal experiment of bacterin
[0069] Use bacterial plate culture, kill bacteria with 1% formaldehyde physiological saline, wash 5 times with sterile physiological saline, detect the concentration of bacterial liquid by spectrophotometer, and then check for viable bacteria, dilute to 1.8 to 2 billion / ml, and make bacteria Seedling.
[0070] The experimental animals used in the animal experiments were 18-20 g mice. Subcutaneous injection 3 times, each injection of 0.3ml. The interval between injections is one week. On the 7th day after the last injection, the cross-agglutinated MSHA antibody titer of the immunized mice was detected.
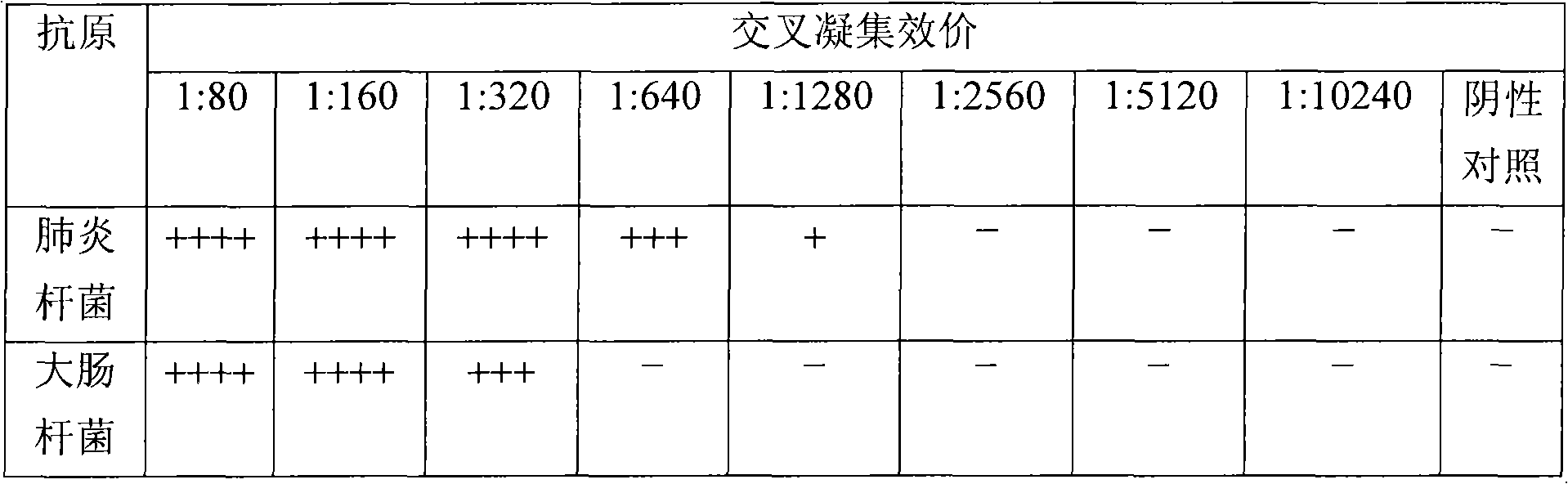
[0071] Table 1. Detection of cross-agglutination titers of antibodies in blood of mice and MSHA antigens of two bacteria after immunization with the "bacterial" of the present invention
[0072]
[0073] A total of 43 mice were immunized successively in the immune protection test. After 17 days of automatic immunization,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com