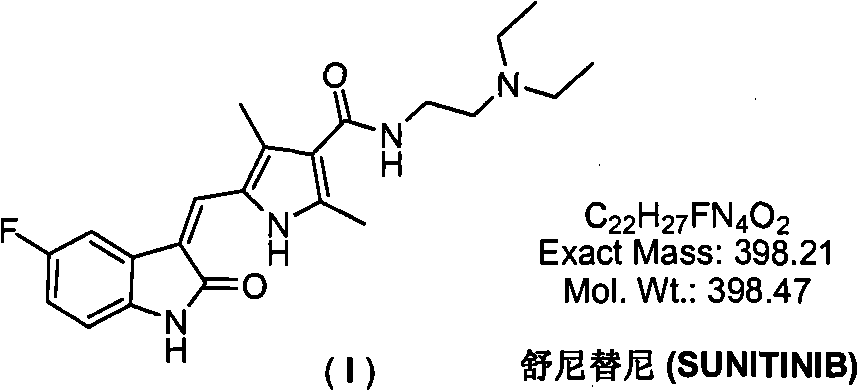
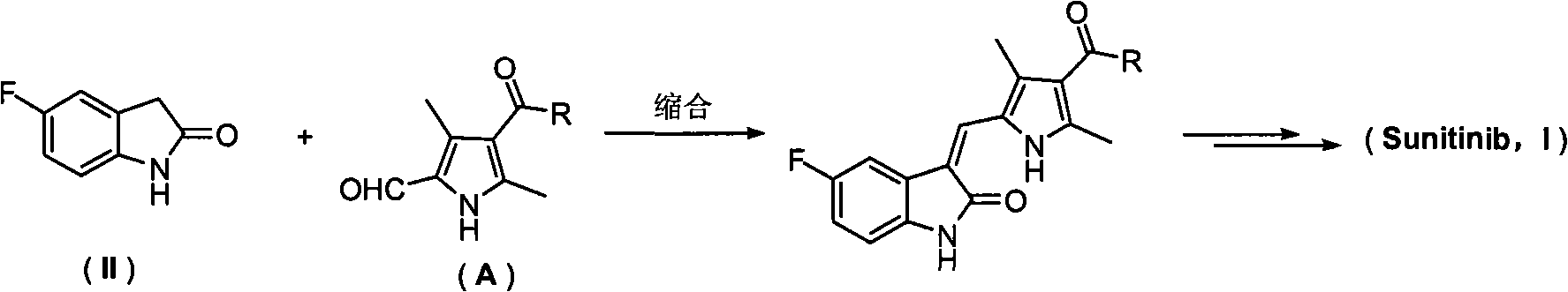
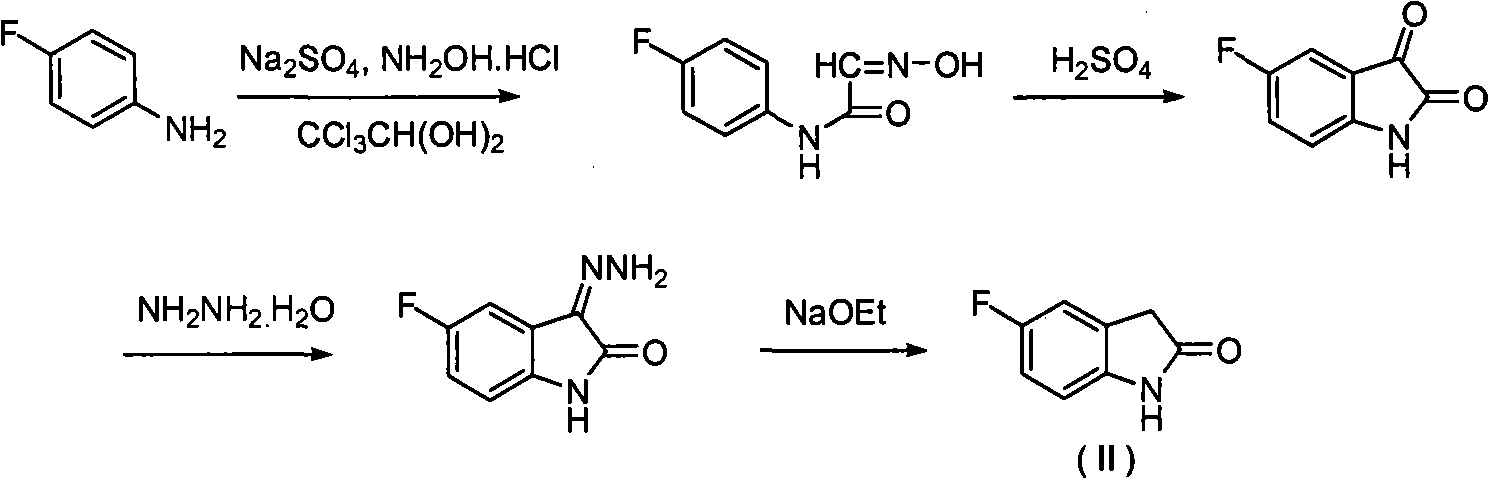
Pyrrolyl acrylamide compound and application thereof to synthesis of sunitinib
A kind of compound, technology of dimethylformamide, applied in the field of preparing sunitinib
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0045] Example 1.2-Chloro-N-(4-fluoro-2-iodo-phenyl)-acetamide (VII-A)
[0046]
[0047] In a 250mL four-neck flask equipped with a drying tube, a thermometer, a dropping funnel and a mechanical stirring paddle, add 4-fluoro-2-iodo-aniline (15.0g, 0.63mol), diisopropylethylamine (8.4g, 0.65mol), and dichloromethane (100mL), stir to dissolve, and cool down. At -10-0°C, a dichloromethane (30 mL) solution of chloroacetyl chloride (7.3 g, 0.64 mol) was added dropwise. After the addition was complete, the temperature was naturally raised and reacted at room temperature until the raw material was completely converted. Wash successively with 2×80 mL saturated brine and 80 mL deionized water, and dry the organic phase over anhydrous sodium sulfate. Filter and remove dichloromethane under reduced pressure. The residue was recrystallized with ethanol, and after vacuum drying, 18.1 g of light yellow flaky crystals were obtained, with a yield of 91.8%. Melting point: 106.6-107.7°C; ...
example 2
[0048] Example 2. Preparation of [(4-fluoro-2-iodo-phenylcarbamoyl)-methyl]-phosphonic acid diethyl ester (IV-A)
[0049]
[0050] In a 100mL three-necked flask equipped with a reflux condenser, a thermometer, and a nitrogen inlet tube, add 2-chloro-N-(4-fluoro-2-iodo-phenyl)-acetamide (VII-A) (11.1g, 35.4mmol), triethyl phosphite (6.1g, 36.5mmol). Under the protection of nitrogen, the reaction was stirred at 130-140° C. for about 16 hours, and TLC showed that the raw materials basically disappeared. After cooling, the obtained reactant was recrystallized with ethyl acetate, and after vacuum drying, 13.6 g of white needle crystals were obtained with a yield of 92.6%. Melting point: 89.1-90.6°C; 12 h 16 FINO 4 P, ESI-MS m / z: [M+H] + 416.04; 1 H NMR (400MHz, DMSO-d6) 9.51(s, 1H), 7.78-7.75(m, 1H), 7.47-7.43(m, 1H), 7.30-7.25(m, 1H), 4.13-4.05(m, 4H), 3.20(d, J=21Hz, 2H), 1.25(t, J=8.0Hz, 3H)ppm; 13 C NMR (100MHz, DMSO-d6) 164.0, 160.8 (d, J C-F =246Hz), 136.5(d, J ...
example 3
[0051] Example 3. Preparation of [(4-fluoro-2-bromo-phenylcarbamoyl)-methyl]-phosphonic acid diethyl ester (IV-B)
[0052]
[0053] According to the method of Example 2, the target compound (IV-B) was prepared from 2-chloro-N-(4-fluoro-2-bromo-phenyl)-acetamide with a yield of 93.6%. Melting point: 112-114°C; 12 h 16 BrFNO 4 P, ESI-MS m / z: [M+H] + 369.04; 1 H NMR (400MHz, CDCl 3 ) = 9.96 (br, 1H), 7.70-7.68 (m, 1H), 7.36-7.32 (m, 1H), 6.85 (t, J = 8.4Hz, 1H), 4.28-4.20 (m, 4H), 3.08 (d, J=21.8Hz, 2H), 1.41(t, J=7.0Hz, 6H); 13 C NMR (100MHz, CDCl 3 )□=162.2(d, J C-P =4.7Hz), 155.5(d, J C-F =242.8Hz), 135.3(d, J C-F = 3.0Hz), 123.9, 119.5 (d, J C-F =6.7Hz), 116.0(d, J C-F =23.1Hz), 108.6(d, J C-F =21.7Hz), 63.3(d, J C-P =6.7Hz), 37.0(d, J C-P =128.9Hz), 16.4(d, J C-P = 6.1 Hz).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com