Aminophosphonate compound, and application thereof in extraction of lithium in alkaline solution containing lithium ions
An aminophosphonate and alkaline solution technology, which can be applied in the fields of compounds of Group 5/15 elements of the periodic table, organic chemistry, and process efficiency improvement, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0109] A method for extracting lithium in an alkaline solution containing lithium ions, the method comprising the steps of:
[0110] 1. Preparation of organic phase: Mix 0.2 mol of extractant with 1.0 L of sulfonated kerosene, and then add sodium hydroxide solution to saponify with a degree of saponification of 30% to obtain an extracted organic phase.
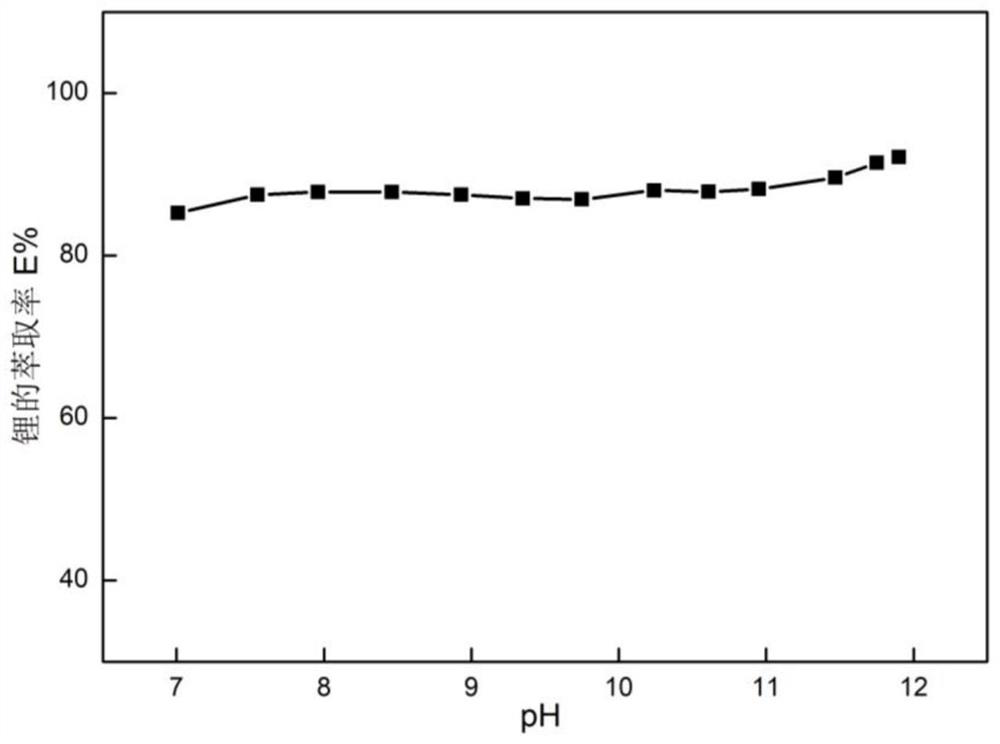
[0111] 2. The original feed solution is the filtrate after lithium precipitation in the salt lake, pH=10.33, and the concentration of each ion is shown in the table
[0112] ion Li Na Mg Ca K g / L 1.85 73.17 0.32 0.039 0.14
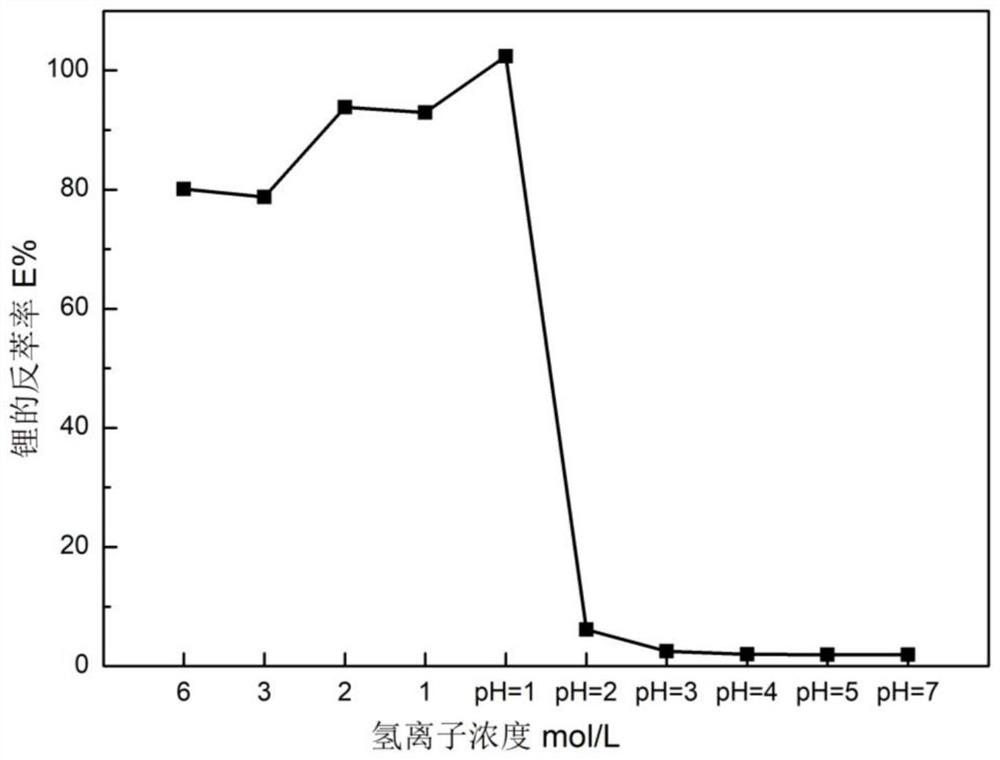
[0113] 3. The washing solution is 0.01mol / L hydrochloric acid solution;
[0114] 4. The back extraction agent is 0.5mol / L hydrochloric acid solution;
[0115] 5. Organic phase regeneration solution: 1mol / L sodium carbonate;
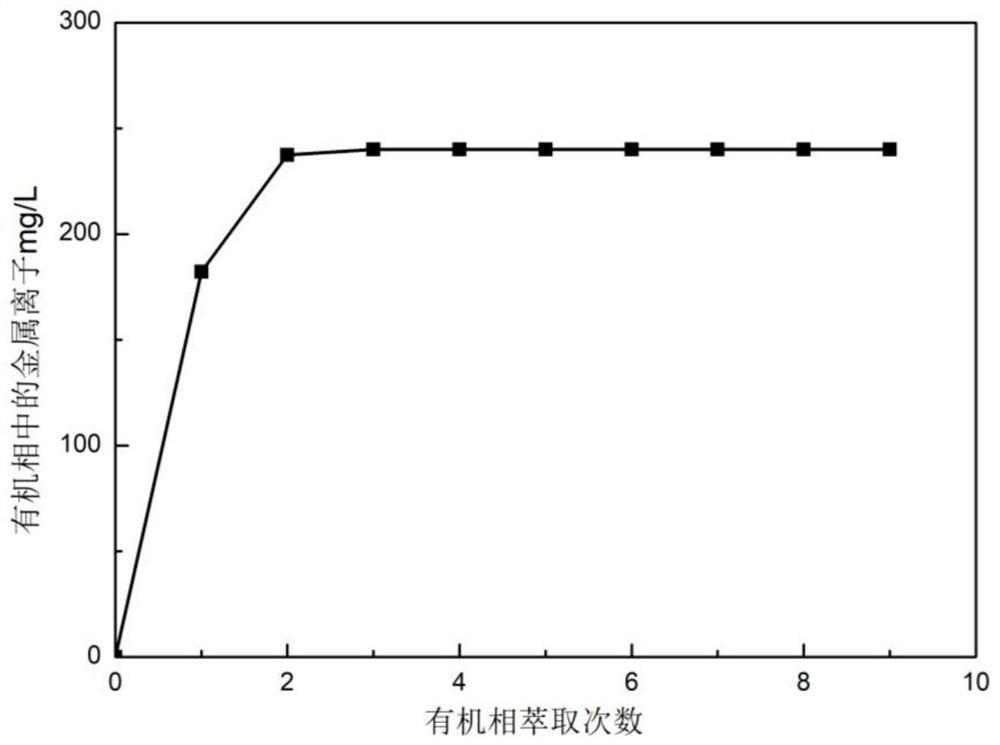
[0116] 6. The organic phase and the feed liquid are subjected to 3-stage countercurrent extraction according to the volume ratio of 8:1 to obtain t...
Embodiment 2
[0120] A method for extracting lithium in an alkaline solution containing lithium ions, the method comprising the steps of:
[0121] 1. Preparation of organic phase: Mix 0.2 mol of extractant with 1.0 L of sulfonated kerosene, and then add sodium hydroxide solution to saponify with a degree of saponification of 30% to obtain an extracted organic phase.
[0122] 2. The experimental feed liquid is the tail liquid after extracting cobalt and nickel from waste ternary batteries, and sodium hydroxide solution is added to adjust the pH of the feed liquid to 9.8. The concentration of each ion is shown in the table:
[0123] ion Li Na Ni Co Mg Ca g / L 1.87 32 0.028 0.005 0.002 0.005
[0124] 3. The washing solution is 0.01mol / L hydrochloric acid solution;
[0125] 4. The back extraction agent is 0.5mol / L hydrochloric acid solution;
[0126] 5. Organic phase regeneration solution: 1mol / L sodium carbonate;
[0127] 6. The organic phase and the feed...
Embodiment 3
[0131] A method for extracting lithium in an alkaline solution containing lithium ions, the method comprising the steps of:
[0132] 1. Preparation of organic phase: Mix 0.3 mol of extractant with 1.0 L of sulfonated kerosene, and then add sodium hydroxide solution to saponify with a degree of saponification of 30% to obtain an extracted organic phase.
[0133] 2. The feed solution is a solution for selective leaching of waste lithium iron phosphate power batteries: the concentration of lithium ions is 3.95g / L, the concentration of iron ions is 0.24g / L, and the pH value of the solution is 4.58. Use sodium hydroxide to adjust the acidity and pH of the feed liquid to be greater than 8, and filter.
[0134] 3. The washing solution is 0.01mol / L hydrochloric acid solution;
[0135] 4. The back extraction agent is 0.5mol / L hydrochloric acid solution;
[0136] 5. Organic phase regeneration solution: 1mol / L sodium carbonate;
[0137] 6. The organic phase and the feed liquid are sub...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com