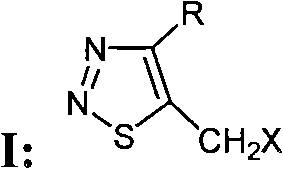
5-halogenated methyl-1,2,3-thiadiazole compound and preparation method and use thereof
A technology of thiadiazoles and halomethyl, which is applied in the field of 5-halomethyl-1, can solve the problems of rare pesticide varieties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
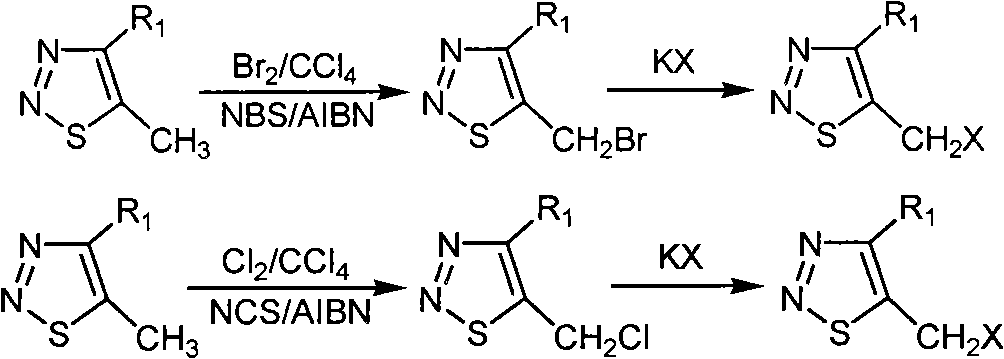
[0047] Preparation and structure identification of compound 5-chloromethyl-1,2,3-thiadiazole-4-carboxylic acid ethyl ester (ZHH-FC)
[0048] 10 ml of carbon tetrachloride was added to a 50 ml round bottom flask, followed by ethyl 5-methyl-1,2,3-thiadiazole-4-carboxylate (1.04 g, 6.05 mmol), N-chloro Succinimide (NCS) (1.27 grams, 7.13 mmol), azobisisobutyronitrile (AIBN) (0.10 grams, 0.61 mmol), the reaction system was refluxed for 6 hours under stirring, after the reaction was completed, filter The product, the solid was washed with 10 ml of carbon tetrachloride × 3, the organic phases were combined, the solvent was evaporated under reduced pressure, and the 200-300 mesh silica gel column chromatography was used to obtain 5-chloromethyl-1,2,3-thiadiazole- 4-Ethyl formate, the eluent is petroleum ether at 60-90 degrees Celsius: ethyl acetate, the volume ratio is 10:1, the yield is calculated with the pure product obtained, and the yield is 13%; carry out 1 Determination of HN...
Embodiment 2
[0050] Preparation and structure identification of the compound 5-bromomethyl-1,2,3-thiadiazole-4-carboxylic acid ethyl ester (ZHH-4)
[0051] Add 10 ml of carbon tetrachloride to a 50 ml round bottom flask, then add 5-methyl-1,2,3-thiadiazole-4-carboxylic acid ethyl ester (1.04 g, 6.05 mmol), N-bromo Succinimide (NBS) (1.27 grams, 7.13 mmol), azobisisobutyronitrile (AIBN) (0.10 grams, 0.61 mmol), the reaction system was refluxed for 6 hours under stirring, after the reaction was completed, filter The product, the solid was washed with 10 ml of carbon tetrachloride × 3, the organic phases were combined, and the solvent was distilled off under reduced pressure to obtain 5-bromomethyl-1,2,3-thiadiazole- 4-Ethyl formate, the eluent is petroleum ether at 60-90 degrees Celsius: ethyl acetate, the volume ratio is 10:1, the yield is calculated with the pure product obtained, and the yield is 24%; carry out 1 Determination of HNMR, 1 HNMR (solvent: CDCl 3 , chemical shift): 5.095 (...
Embodiment 3
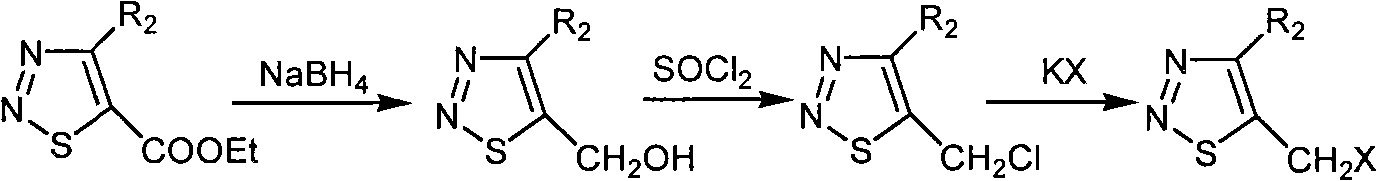
[0053] Preparation and structure identification of compound 5-iodomethyl-1,2,3-thiadiazole-4-carboxylic acid ethyl ester (ZHH-14)
[0054] Add 5-bromomethyl-1,2,3-thiadiazole-4-carboxylic acid ethyl ester (0.20 g, 0.8 mmol) in a 50 ml round bottom flask, add 15 ml solvent acetone, then add potassium iodide (0.20 g , 1.2 mmol), stirred at room temperature for 20 hours, filtered out the solid in the solution, evaporated the solvent under reduced pressure and obtained 5-iodomethyl-1,2,3-thiadiazole- with 200-300 mesh silica gel column chromatography 4-Ethyl formate, the eluent is petroleum ether at 60-90 degrees Celsius: ethyl acetate, the volume ratio is 5: 1, the yield is calculated with the pure product obtained, the yield is 59%, and the 1 Determination of HNMR, 1 HNMR (solvent: CDCl 3 ), chemical shift: 5.089 (s, 2H, CH 2 ), 4.578-4.525 (q, 2H, J=7.2Hz, J=14.4Hz, CH 2 ), 1.515-1.479 (t, 3H, J=7.2Hz, CH 3 ), the compound's 1 The H NMR data show that its chemical structu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com