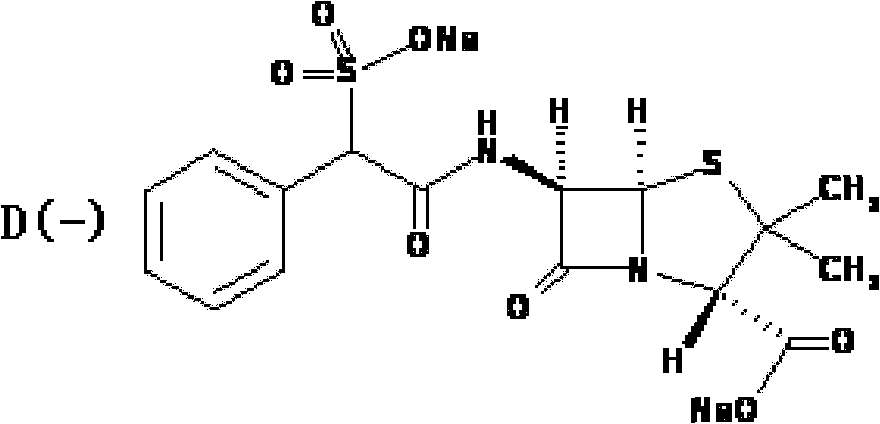
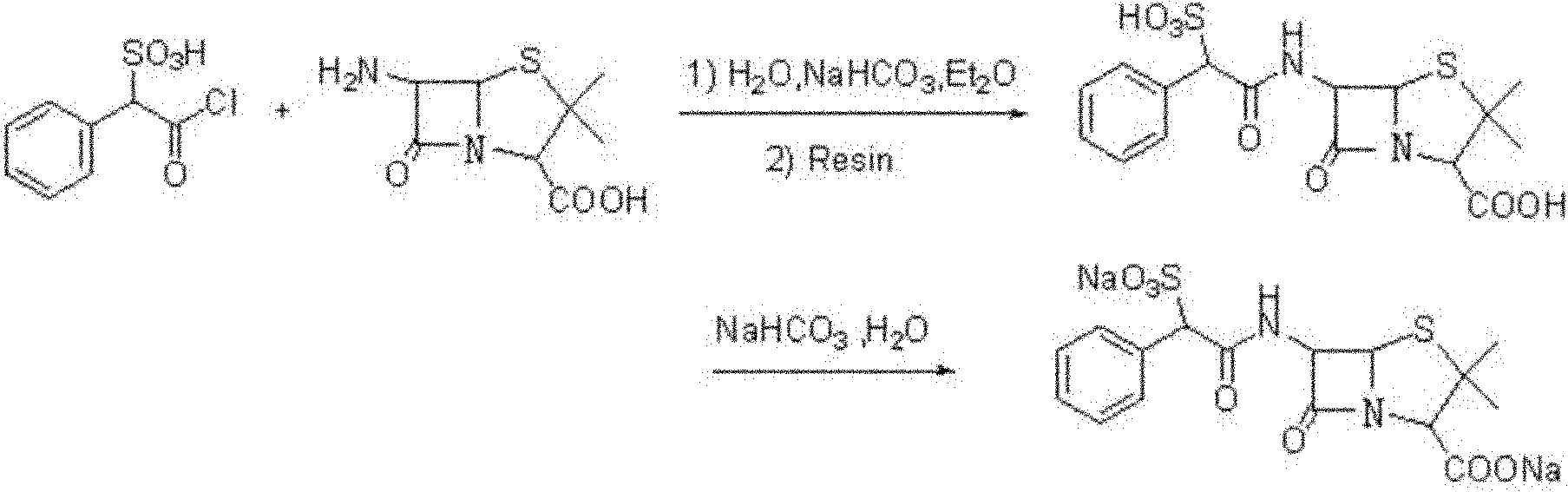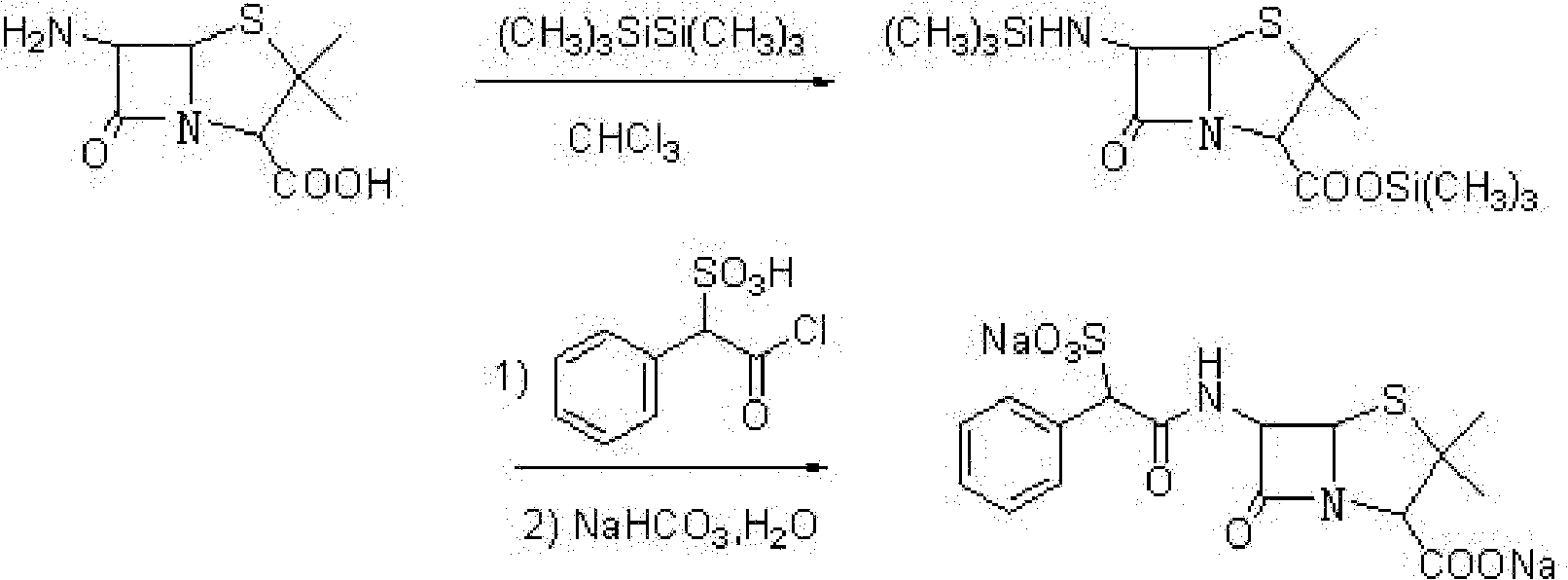Preparation method of D(-)-sulbenicillin sodium
A technology of sulfobenicillin sodium and sulfobenicillin, applied in the direction of organic chemistry and the like, can solve the problems of harsh operating conditions, long reaction time, unsatisfactory yield and the like, and achieve the effects of simple operation, improved reaction temperature and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Stir and dissolve 52.4g (0.24mol) of D(-)-sulfophenylacetic acid in 100mL of ether, add 160mL of thionyl chloride dropwise at -5°C, add dropwise 1.6ml of N,N-diisopropylethylamine, Then, the reaction was stirred and reacted for 2.0 hours at a temperature of 25°C. After the reaction was completed, it was distilled to dryness under reduced pressure, and the residue after distillation was washed twice with ether, and then distilled to dryness under reduced pressure to obtain 58.6 g of D(-) -sulfophenylacetyl chloride, yield 81%;
[0047]Add 20.5g of 6-APA (0.095ml) into a mixed solvent of 50mL of water, 34mL of ethanol and 8.5mL of 2-methyltetrahydrofuran, keep the temperature at 20°C, and then add dropwise a 10% sodium hydroxide solution to adjust the pH to 5.6 , stirred at 20°C until the solids were completely dissolved, then added dropwise an ethyl acetate solution containing 30.0g D(-)-sulfophenylacetyl chloride at this temperature, after the dropwise addition was comp...
Embodiment 2
[0050] Stir and dissolve 52.4g (0.24mol) of D(-)-sulfophenylacetic acid in 100mL of ether, add 89mL of thionyl chloride dropwise at -3°C, add dropwise 1.2ml of N,N-diisopropylethylamine, Then, the reaction was stirred and reacted for 2.5 hours at a temperature of 20°C. After the reaction was completed, distilled to dryness under reduced pressure, and repeated twice to wash the residue after distillation with ether, and then distilled to dryness under reduced pressure to obtain 60.1gD(-) -sulfophenylacetyl chloride, yield 83%;
[0051] 20.5g (0.095ml) of 6-APA was added to a mixed solvent of 50mL of water, 25mL of ethanol and 12.5mL of 2-methyltetrahydrofuran, and the temperature was kept at 25°C, and then a 15% sodium hydroxide solution was added dropwise to adjust the pH to 6.0, Stir at 25°C until the solid is completely dissolved, then add dropwise an ethyl acetate solution containing 34.0g D(-)-sulfophenylacetyl chloride at this temperature, after the addition is complete, ...
Embodiment 3
[0054] Stir and dissolve 52.4g (0.24mol) of D(-)-sulfophenylacetic acid in 100mL of ether, add 115mL of thionyl chloride dropwise at 0°C, add dropwise 2.0ml of N,N-diisopropylethylamine, and then Stir the reaction at a temperature of 20°C for 1.5 hours. After the reaction is complete, distill under reduced pressure to dryness, repeat 3 times to wash the residue after distillation with ether, and then distill to dryness under reduced pressure to obtain 57.7g D(-) -sulfophenylacetyl chloride, yield 80.5%;
[0055] Add 20.5g of 6-APA (0.095ml) into a mixed solvent of 50mL of water, 29mL of ethanol and 10mL of 2-methyltetrahydrofuran, keep the temperature at 15°C, and then add dropwise a 10% sodium hydroxide solution to adjust the pH to 7.0, Stir at 15°C until the solid is completely dissolved, and add dropwise an ethyl acetate solution containing 31.2 g of D(-)-sulfophenylacetyl chloride at this temperature, wherein the ethyl acetate solution of D(-)-sulfophenylacetyl chloride is...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com