Long life lithium batteries with stabilized electrodes
A technology of electrodes and stabilizing additives, used in lithium batteries, non-aqueous electrolyte batteries, battery electrodes, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
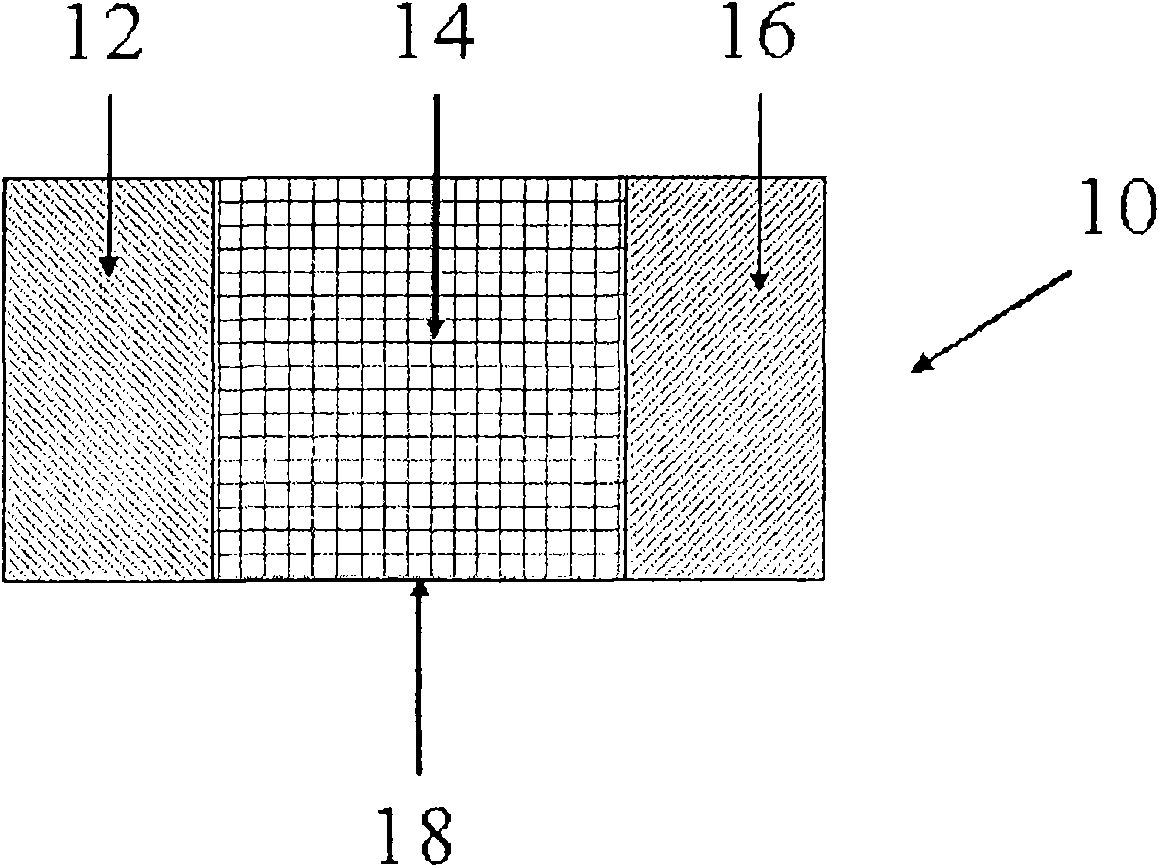
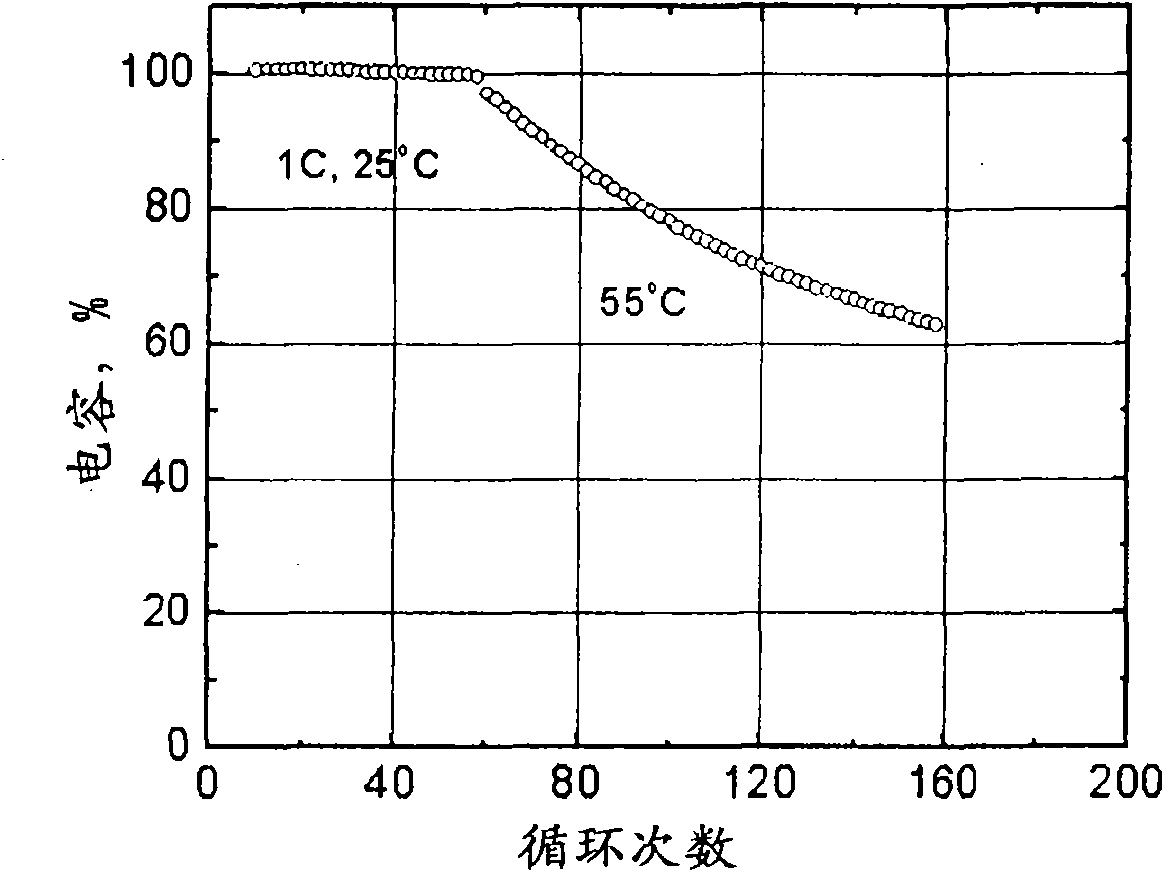
[0088] EXAMPLE 1 This particular example referred to here utilizes an electrochemical cell such as figure 1 The electrochemical cell shown. refer to figure 1 , electrochemical cell 10 includes an anode 12 and a cathode 16 separated by an electrolyte / separator 14 , all housed within an insulating housing 18 . The anode is separated from the cathode by an electrolyte and suitable terminals (not shown) are provided to make electrical contact with the anode 12 and cathode 16 respectively. Binders (eg, polyvinylidene fluoride) associated with each electrode are well known in the art and will not be described here. In this particular embodiment, the electrochemical cell comprises a graphite anode, such as natural graphite, artificial graphite, meso-carbon microspheres, carbon fibers, or hard carbon, a manganese spinel cathode, and About 1.2M LiPF in 3:7 by weight 6 electrolyte. figure 2 Depicts the resulting capacity retention when the battery is cycled between 3.0 and 4.1V. ...
Embodiment 2
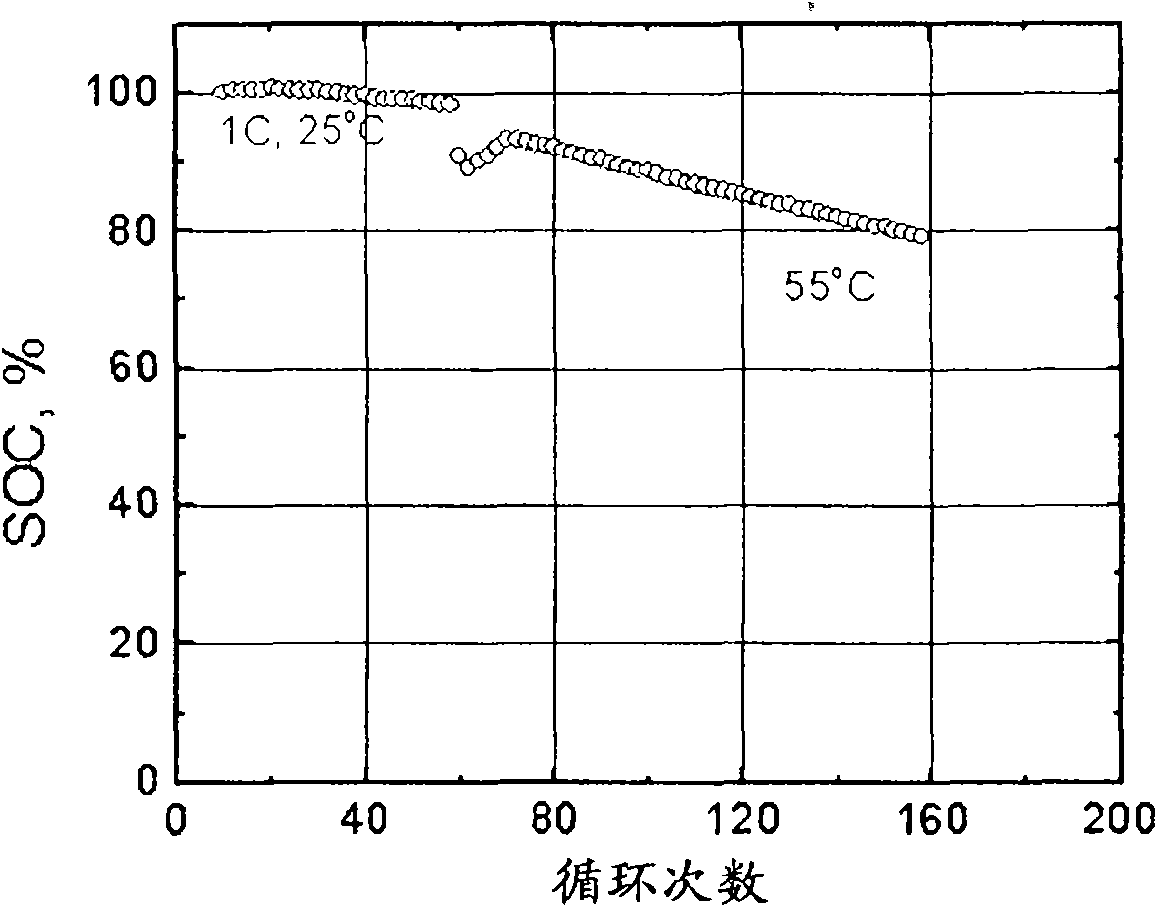
[0089] Example 2 1 wt % 3,9-divinyl-2,4,8,10-tetraoxaspiro[5.5]undecane (TOS-1, available from SIGMA-ALDRICH, Milwaukee) was added to Example 1 in the electrolyte of an electrochemical cell. Then, the cell was cycled at 55°C for more than 150 cycles at 100% DOD. image 3 The results of cycling the battery between 3.0 and 4.1V are shown. This cell demonstrates improved capacity retention compared to the electrochemical cell of Example 1 (compare figure 2 and 3 ). It is believed that this improvement comes from the formation of a thin film on the electrode by the additive. Figure 4 The results of cyclic voltammetry with TOS-1 are shown over the range of voltages encountered within the electrochemical cell. The increase in current is consistent with oxidation and / or polymerization of the additive.
Embodiment 3
[0090] Example 3 In order to study that although Mn 2+ The dissolution of ions was suppressed, but the reason for the significant degradation of the graphite / substituted spinel cell was that the AC impedance of the cell was measured using a specially designed Li-Sn reference electrode during cycling at 55 °C. Figure 5 Results are shown. After one formation cycle at room temperature [ Figure 5 A], and after 25 cycles at 55 °C [ Figure 5 B] Measure the AC impedance. In the initial stage of cycling, the impedance of the negative electrode was much smaller than that of the positive electrode; however, after 25 cycles at 55 °C, the impedance of the negative electrode increased significantly and overwhelmingly surpassed that of the positive electrode.
[0091] Graphite anodes cycled in manganese spinel-based Li-ion cells at 55°C were examined by energy dispersive spectroscopy (EDS). The EDS spectrum clearly shows the presence of Mn metal on the graphite surface. It is believ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com