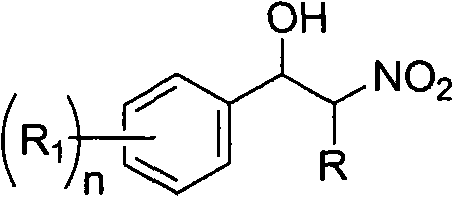
Application of polyhydroxy fullerene for catalyzing addition reaction of nitroparaffin and aromatic aldehyde
A technology of polyhydroxyfullerene and hydroxyfullerene is applied in the formation/introduction of hydroxyl groups, chemical instruments and methods, preparation of organic compounds, etc., to achieve the effects of high catalytic efficiency, high catalytic selectivity, and long catalytic life.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Add 10mL tetrahydrofuran, 2mmol (107μL) nitromethane and 0.5mmol (75mg) 4-nitrobenzaldehyde in a 25mL single-necked bottle, then add 10mg polyhydroxy fullerene catalyst, and react with magnetic stirring at 25°C for 10 hours, The nitroalcohol product represented by formula III is obtained.
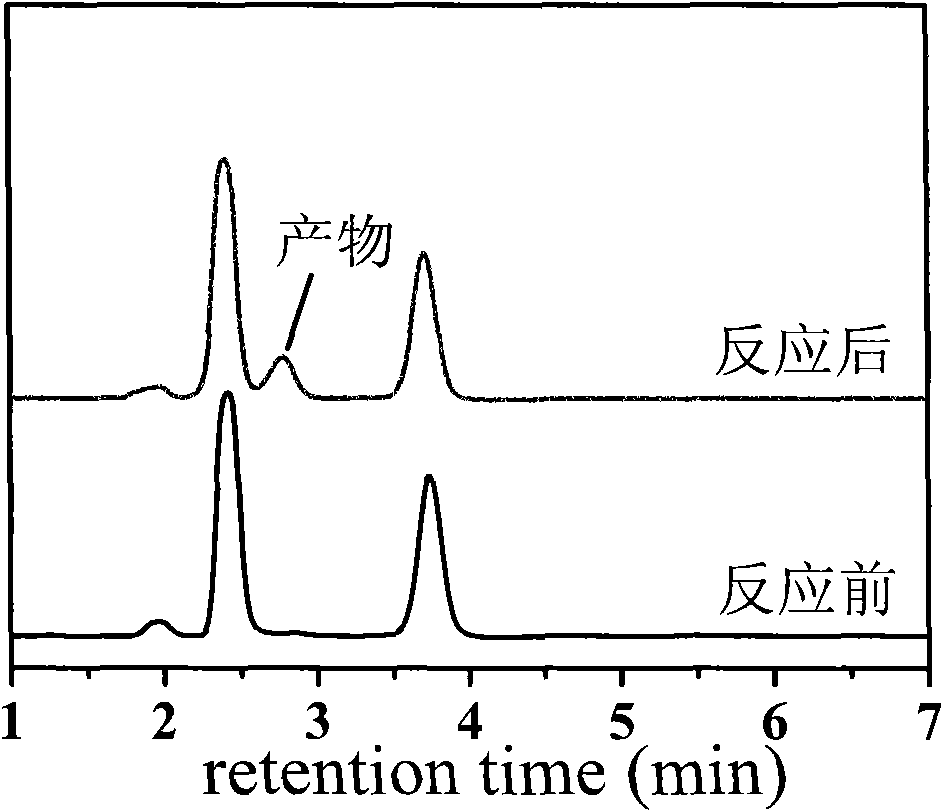
[0041] Get the mixture in the bottle and centrifuge to remove the catalyst, and the supernatant is analyzed by high-performance liquid chromatography. The analysis conditions are as follows: methanol: water=7: 3 (V: V), flow rate: 1ml / min, ultraviolet detection wavelength: 200nm, chromatographic Column: reverse phase C18 column, column temperature: 25°C. The result is as figure 1 shown. Depend on figure 1 It can be seen that the conversion rate of nitromethane is 98%, the yield of nitroalcohol product is 97%, and the selectivity is 100%.
[0042] In order to verify the correctness of the structure of the obtained nitroalcohol product, the supernatant obtained by centrifugation co...
Embodiment 2
[0047] Add 10mL tetrahydrofuran, 2mmol (107μL) nitromethane and 0.5mmol (65.6mg) 4-cyanobenzaldehyde into a 25mL single-necked bottle, then add 10mg polyhydroxy fullerene catalyst, and react with magnetic stirring at 25°C for 10 hours , to obtain the nitroalcohol product shown in formula III.
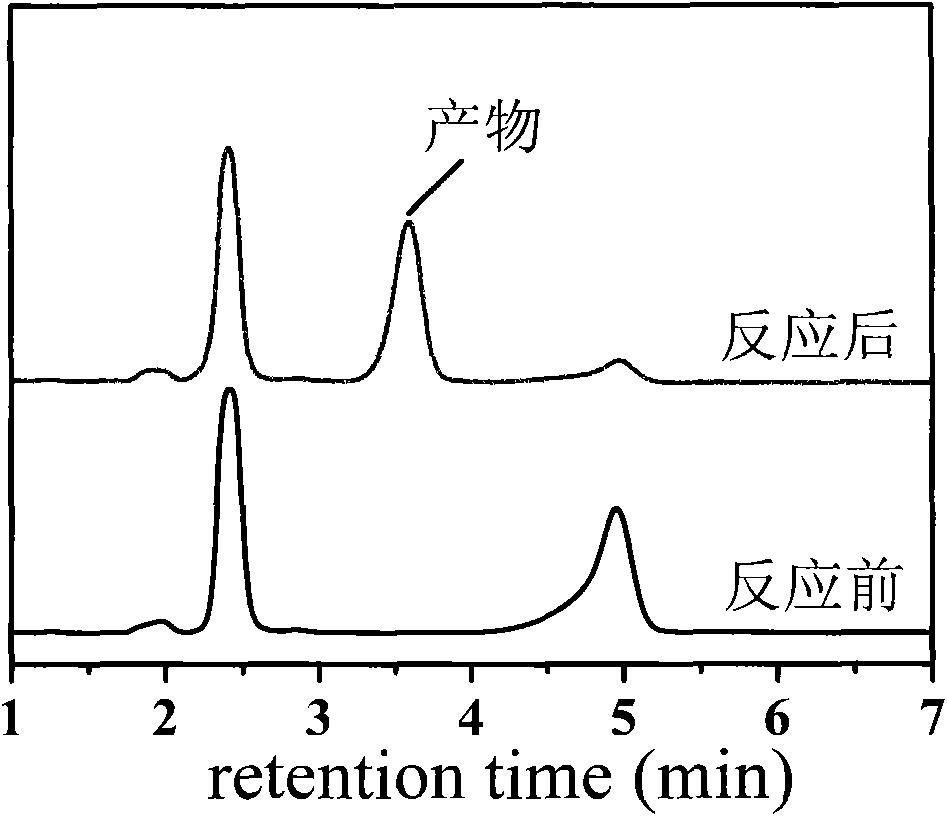
[0048] Get the mixture in the bottle and centrifuge to remove the catalyst, and the supernatant is analyzed by high-performance liquid chromatography. The analysis conditions are as follows: methanol: water=6: 4 (V: V), flow rate: 1ml / min, ultraviolet detection wavelength: 200nm, chromatographic Column: reverse phase C18 column, column temperature: 25°C. The result is as figure 2 shown. Depend on figure 2 It can be seen that the conversion rate of nitromethane is 99%, the yield of nitroalcohol product is 98%, and the selectivity is 100%.
[0049] In order to verify the correctness of the structure of the obtained nitroalcohol product, the supernatant obtained by centrifugation con...
Embodiment 3
[0054] Add 10mL of dichloromethane, 2mmol (107μL) of nitromethane and 0.5mmol (68.3μL) of 4-trifluoromethylbenzaldehyde into a 25mL single-necked bottle, then add 10mg of polyhydroxy fullerene catalyst, and magnetically The reaction was stirred for 10 hours to obtain the nitroalcohol product represented by formula III.
[0055] Get the centrifugation of the mixture in the bottle to remove the catalyzer, and the supernatant is analyzed by high-performance liquid chromatography. The analysis conditions are the same as in Example 1, and the obtained results are as follows: image 3 shown. Depend on image 3 It can be seen that the conversion rate of nitromethane is 98%, the yield of nitroalcohol product is 97%, and the selectivity is 100%.
[0056] In order to verify the correctness of the structure of the obtained nitroalcohol product, the supernatant obtained by centrifugation continued to be analyzed by mass spectrometry (EI ion source, ion source temperature: 200 ° C), and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com