Metal complex and preparation and application thereof
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Synthesis of ligand compounds:
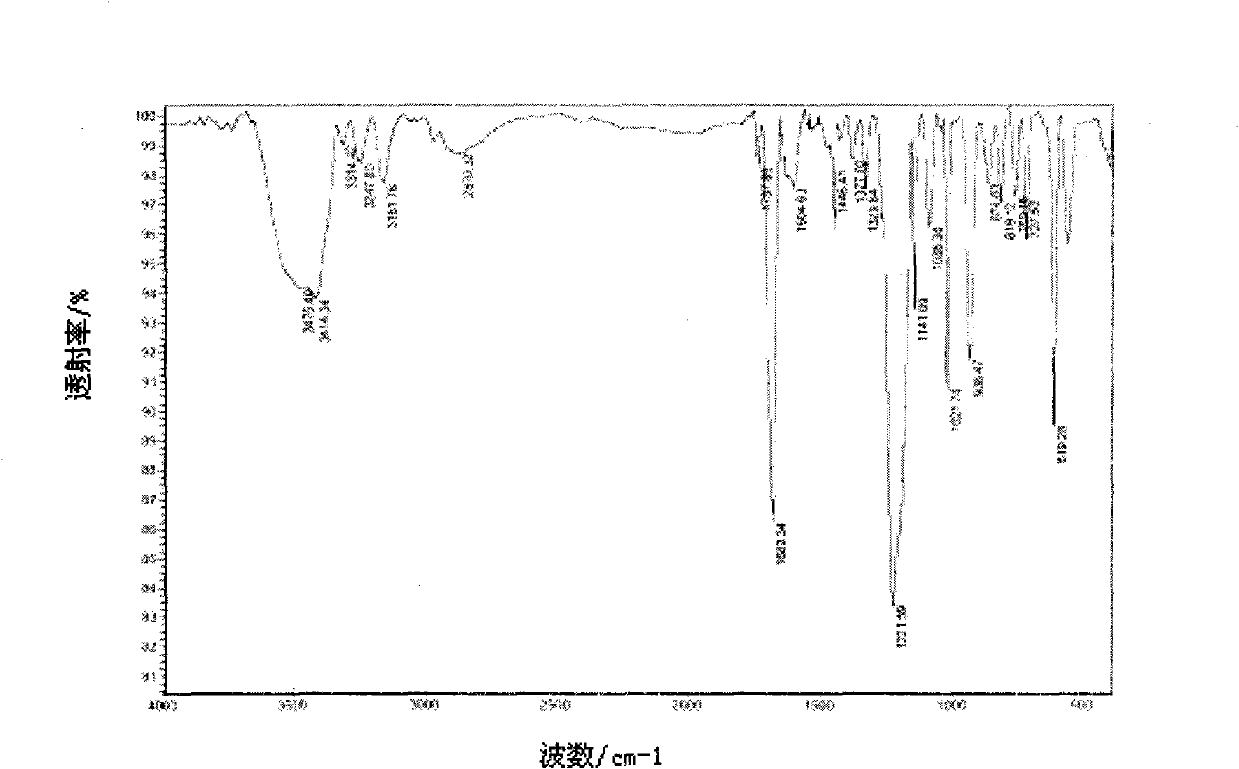
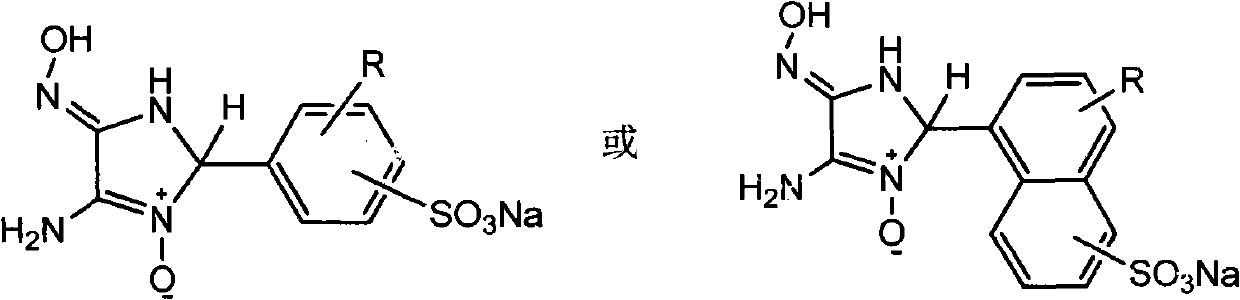
[0030] Add 0.354g (0.003mol) of 3,4-diaminoglyoxime and 50mL of absolute ethanol to a 100mL three-necked flask, heat to about 50℃ to dissolve, add 0.687g (0.0033mol) of sodium o-formylbenzene sulfonate and 0.0855 g (0.00045mol) p-toluenesulfonic acid, reacted for 18 hours, filtered, washed, dried and weighed to obtain 0.8312 g of white solid, yield 85%, melting point: 232-233°C (decomposition). See attached figure 1 , It is the nuclear magnetic resonance spectrum of the ligand compound prepared according to the technical scheme of this embodiment; see attached image 3 , Which is the infrared spectrum of the ligand compound prepared according to the technical scheme of this embodiment; the ligand compound is: 1 H-NMR(400MHz, DMSO-d 6 )δ ppm : 6.78 (s, 1H), 6.92 (s, 1H), 7.12 (dd, J = 7.18, 0.83 Hz, 1H), 7.39 (m, 2H), 7.79 (dd, J = 7.36, 1.52 Hz, 1H), 10.06(s, 1H); IR(KBr, cm -1 ): 3475, 3414 (NH 2 ), 3247, 3161 (OH), 2870 (imidazole ring)...
Embodiment 2
[0040] See Example 1 for the synthesis of ligand compounds.
[0041] Preparation of metal complex:
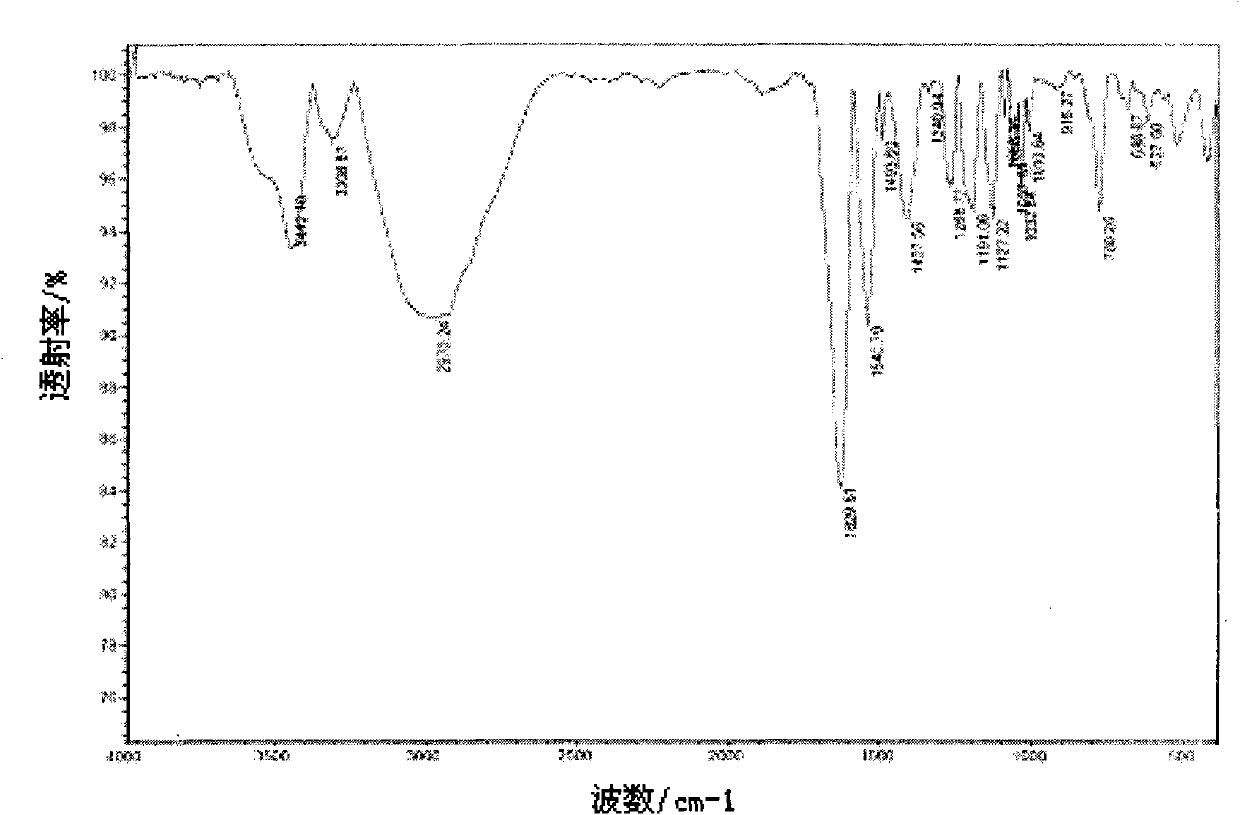
[0042] 1.4792 g (0.0048 mol) of the ligand compound and 30 mL of water were added to a 100 mL three-necked flask and reacted for 5 hours to obtain 1.2328 g of a light brown solid with a yield of 65%. Melting point: greater than 250°C. See attached Image 6 , It is the infrared spectrum of the metal complex prepared according to the technical scheme of this embodiment; the metal complex is: IR (KBr, cm -1 ): 3419(NH 2 ), 3317, 3253, 3170 (OH), 2920 (N=CH), 1707 (OH...O), 1679 (C=N), 1637, 1540, 1446 (benzene ring skeleton), 1393, 1324 (N=O ), 1221, 1160 (CN; SO), 1088 (SO), 1018, 938 (NO), 871, 760 (benzene ring CH).
[0043] Dyeing method:
[0044] Use the above-prepared metal complex as a metal complex dye, with a dosage of 4% owf, add sodium sulfate 30 g / l, bath ratio 1:90, adjust the pH to 2.5 with a buffer solution, and set the 11206 silk at 70°C. Dyeing with electric power spin...
Embodiment 3
[0046] Synthesis of ligand compounds:
[0047] Add 0.7080g (0.006mol) of 3,4-diaminoglyoxime and 300mL of anhydrous methanol to a 500mL three-necked flask, heat up to about 55℃ to dissolve, and add 1.715g (0.0072mol) of 4-methoxybenzaldehyde- Sodium 3-sulfonate and 0.342 g (0.0018 mol) p-toluenesulfonic acid were reacted for 20 hours, filtered, washed, dried, and weighed to obtain 1.7937 g of light yellow solid with a yield of 84%. Melting point: 226-228°C (decomposition). See attached figure 2 , It is the nuclear magnetic resonance spectrum of the ligand compound prepared according to the technical scheme of this embodiment; see attached Figure 4 , Which is the infrared spectrum of the ligand compound prepared according to the technical scheme of this embodiment; the ligand compound is: 1 H-NMR(400MHz, DMSO-d 6 )δ ppm : 3.74 (s, 3H), 6.02 (s, 1H), 6.98 (d, J = 8.58 Hz, 1H), 7.32 (dd, J = 8.26, 2.28 Hz, 1H), 7.64 (d, J = 2.30 Hz, 1H), 8.80 (s, 1H), 9.50 (s, 2H), 10.84 (s, 1H...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com