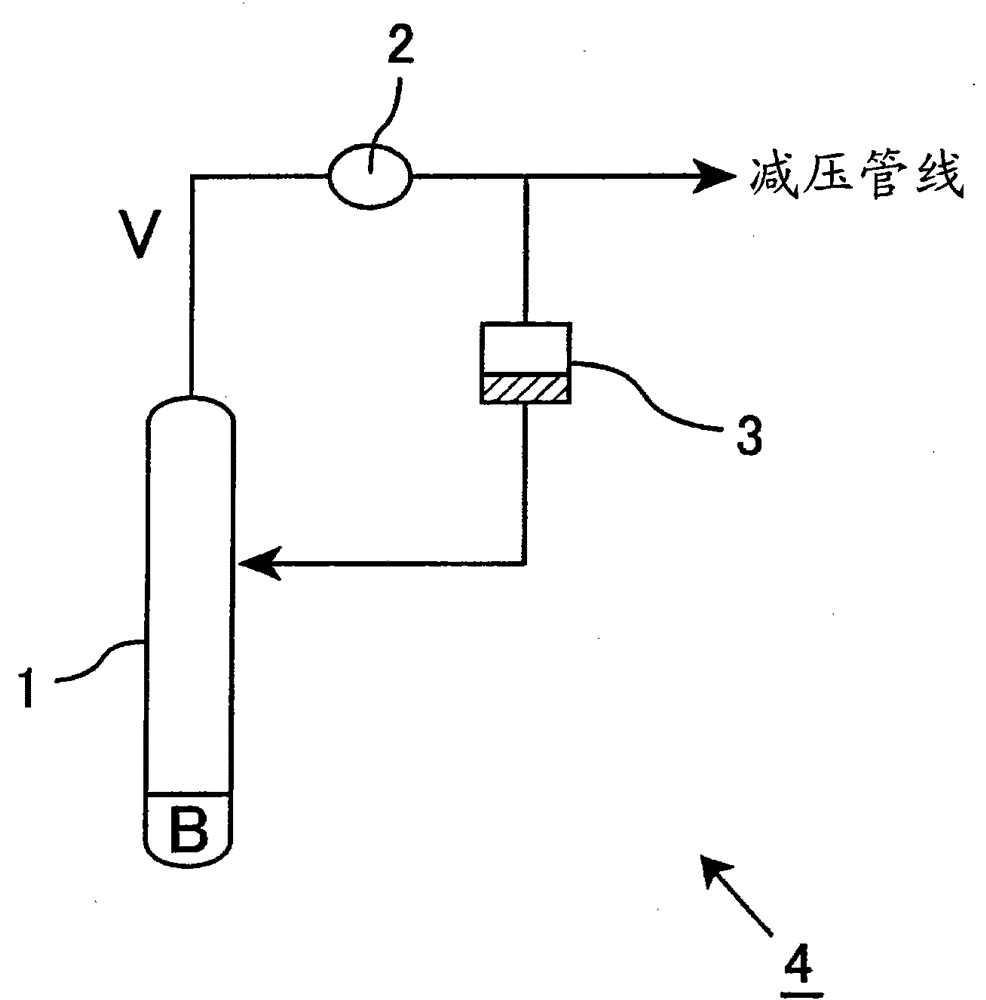
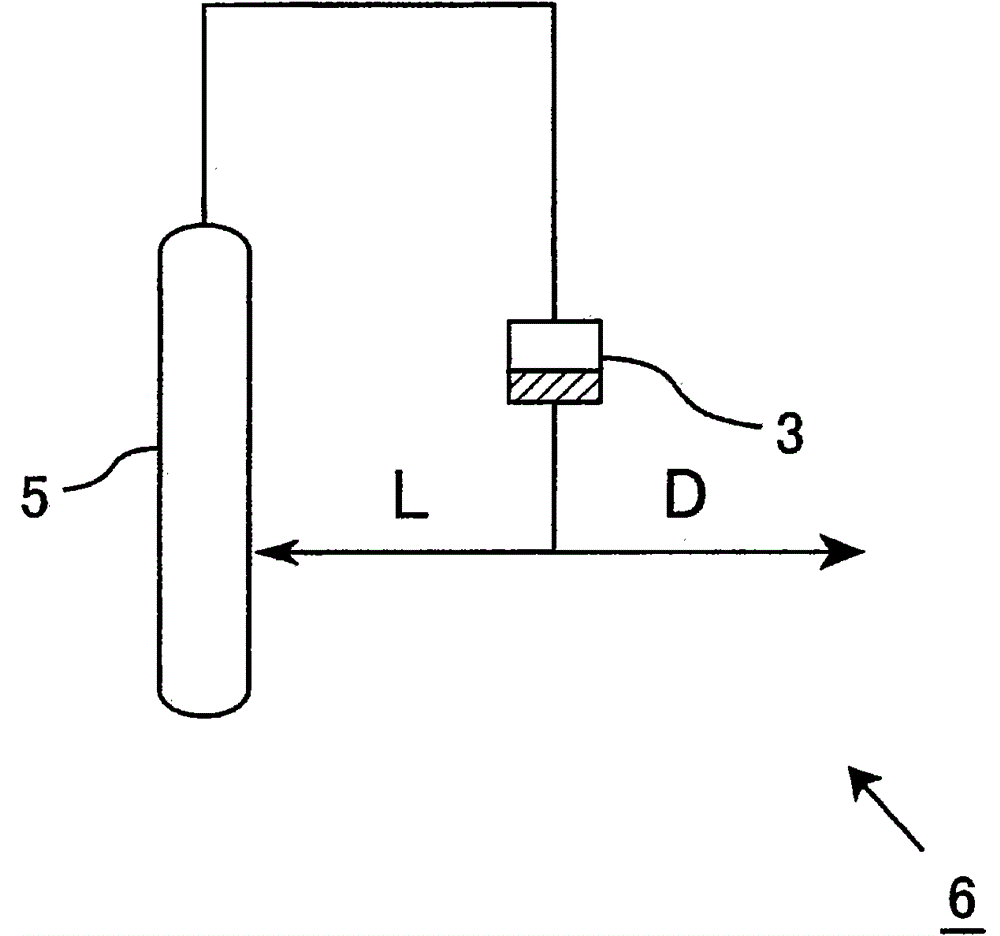
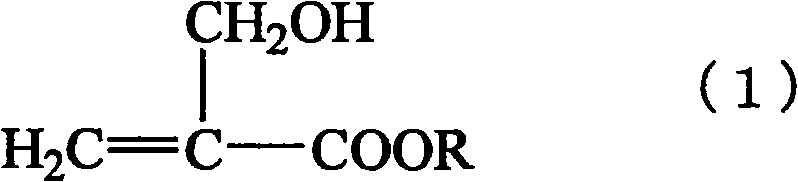α-Hydroxymethyl acrylate compounds and production method thereof
A technology of methyl hydroxymethyl acrylate and a manufacturing method, applied in the field of α-hydroxymethyl acrylate compounds and their manufacture, can solve the problems such as the inability to fully remove formaldehyde, and achieve the effect of preventing pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Synthetic example 1
[0168] (Synthesis example 1 (synthesis of crude RHMAs))
[0169] 2066 g (24 moles) of methyl acrylate as an acrylate, 195.8 g (6 moles in terms of formaldehyde) as formaldehyde were added to a 3-L four-neck flask equipped with a thermometer, a gas blowing pipe, a cooling pipe, a stirring device, and a water bath. 92% by mass of paraformaldehyde as a raw material, 237.8 g (1.2 mol) of a 30% by mass trimethylamine aqueous solution as a catalyst, and 2.1 g of p-methoxyphenol as a polymerization inhibitor. The ratio of p-methoxyphenol to methyl acrylate was 1000 ppm. Thereafter, while blowing air into the reaction solution, the reaction solution was stirred at 70° C. for 8 hours to perform a reaction.
[0170]After the reaction, the reaction solution was transferred to a separatory funnel and separated into an organic phase and an aqueous phase. Then, 100 g of water was added to the organic phase for water washing. After being separated into an organic phase and an aqueous phas...
Embodiment 1
[0173] Transfer 600 g of the crude RHMA obtained in Synthesis Example 1 into a four-neck flask with a capacity of 2 L equipped with a thermometer, a gas blowing tube, an empty column distillation tube, a stirring device, and an oil bath, and keep the internal temperature at 110 to 120° C. while blowing in air. , The condition of pressure 20hPa (15mmHg) was refluxed for 10 hours. The liquid distilled at this time was cooled at 10 degrees by a condenser and returned to the flask, and the gas component containing formaldehyde was discharged into the vacuum system.
[0174] The cumulative steam amount at this time was 801 g, the total reflux ratio was 1.3, and the formaldehyde concentration in the obtained crude RHMAs was 320 ppm.
[0175] The crude RHMAs obtained by the above-mentioned method were subjected to fractional distillation by the method described in Comparative Example 1 described later to obtain 407 g of RHMA-M having a formaldehyde concentration of 20 ppm. The resul...
Embodiment 2~5
[0177] In Example 1, the reflux time was changed to 8 hours (Example 2), 6 hours (Example 3), 5 hours (Example 4) or 4 hours (Example 5), and the polymerization inhibitor was changed to two Copper butyldithiocarbamate (Example 2), 2,2,6,6-Tetramethylpiperidinyl radical (TEMPO) (Example 3), except that RHMA was obtained in the same manner as in Example 1 -M. Table 1 shows the results of total reflux ratio, crude RHMAs and formaldehyde concentration in purified RHMA-M.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com