Method for producing paratyphus living vaccine for piglets
A technology for piglet paratyphoid fever and production methods, which is applied in the direction of medical formulas, medical preparations containing active ingredients, non-active ingredients of polymer compounds, etc., and can solve the problems of large influence of type, age, freshness and degree of digestion, low survival rate, Issues such as instability between batches
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
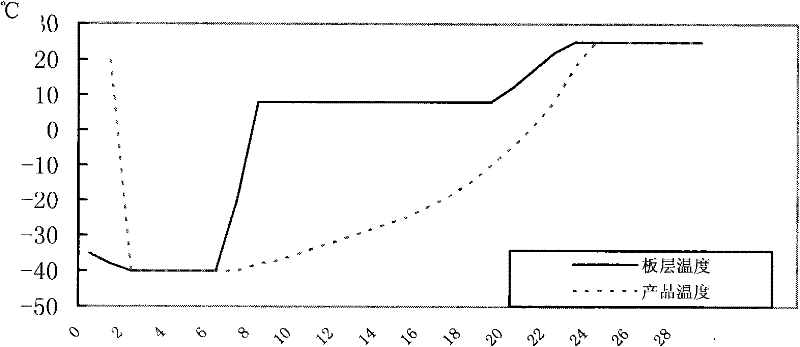
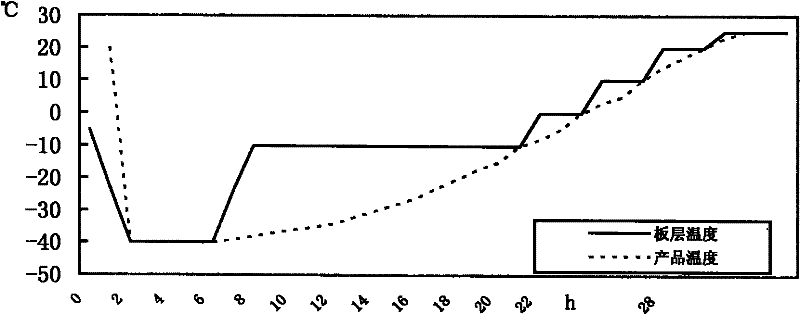
[0054] Inoculate the seed solution of Salmonella choleraesuis (CVCC79500 strain) in the synthetic medium (liquid medium) provided by the present invention according to 1% to 2% of the total medium amount, and ferment or aerate for 18~21h at 37°C. Add an appropriate amount of antifoaming agent as needed, and add an appropriate amount of sterilized 40% glucose to control the pH value according to the increase in pH. After the cultivation, centrifuge at 3500r / min for 20min, remove the supernatant, immediately add the sterilized and preheated to 37°C heat-resistant freeze-drying protective agent provided by the present invention, mix well, and dispense according to the regulations. Should pay attention to insulated and shaken evenly in the subpackaging process, and carry out freeze vacuum drying rapidly, press the method that " Chinese Veterinary Pharmacopoeia " stipulates, use freeze-drying curve 2 that the present invention provides (see figure 2 ), the number of live bacteria i...
Embodiment 2
[0056] Inoculate the seed liquid of Salmonella choleraesuis (strain CVCC79500) in common broth medium (formulation recorded in "Chinese Veterinary Pharmacopoeia") according to 1% to 2% of the total medium, ferment or aerate at 37°C for 18 to 21 hours, During the cultivation process, an appropriate amount of antifoaming agent was added as needed, and an appropriate amount of sterilized 40% glucose was added according to the pH rise to control the pH value. After the cultivation, centrifuge at 3500r / min for 20min, remove the supernatant, immediately add the sterilized and preheated heat-resistant protective agent to 37°C, mix thoroughly, and dispense according to the prescribed portion. Should pay attention to insulated and shaken evenly in the subpackaging process, and carry out freeze vacuum drying rapidly, press the method that " Chinese Veterinary Pharmacopoeia " stipulates, use freeze-drying curve 2 that the present invention provides (see figure 2 ), the number of live bac...
Embodiment 3
[0058] Product testing
[0059] Appearance White spongy loose mass, easy to separate from the bottle wall, dissolve quickly after adding diluent.
[0060] The pure test shall be tested according to the method stipulated in the "Chinese Veterinary Pharmacopoeia", and it shall be pure.
[0061] The number of live bacteria should be marked according to the bottle label, and the number of live bacteria should be counted on an ordinary agar plate (according to the method stipulated in the "Chinese Veterinary Pharmacopoeia"). The number of live bacteria in each head should not be less than 3×10 9 CFU.
[0062] Safety inspection According to the label on the bottle, mark the dose, dilute the vaccine with ordinary broth or peptone water, inject subcutaneously into 2 rabbits weighing 1.5-2.0kg, each 1.0ml (including 2 doses), observe for 21 days, and should survive.
[0063] The remaining moisture shall be determined according to the method stipulated in "Chinese Veterinary Pharmacop...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com