Preparation method of 1,1,1,3,3-pentafluorobutane
A technology for pentafluorobutane and pentachlorobutane, which is applied in 1 field, can solve problems such as poor mass transfer and heat transfer effects, and achieve the effects of accelerating the reaction speed, increasing the reaction temperature and increasing the reaction speed.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
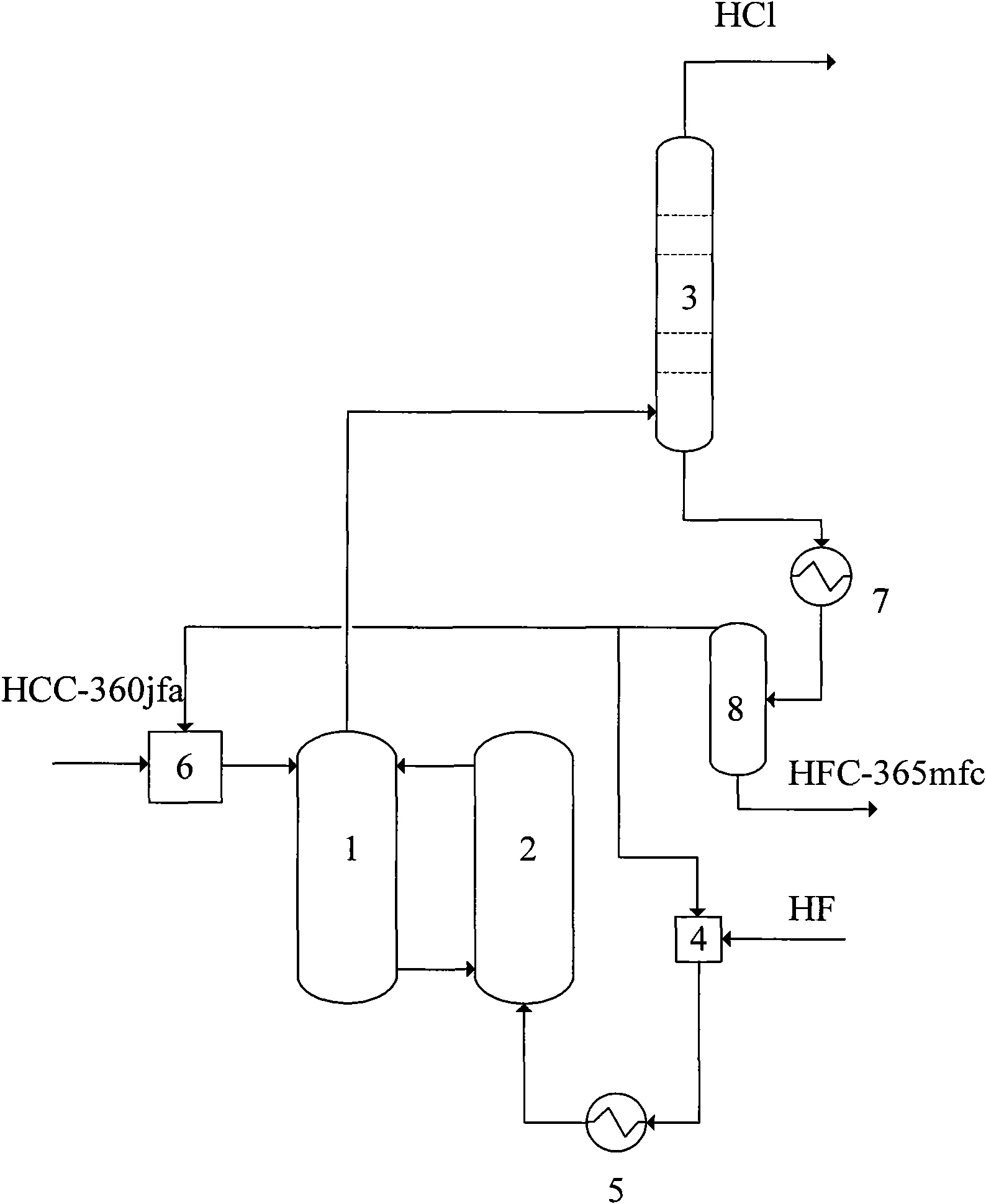
[0026] The temperature of the low temperature reaction zone (1) of the liquid phase fluorination reactor is controlled at 90°C, the temperature of the low temperature reaction zone (1) is controlled at 140°C, and the HF entering the liquid phase fluorination reactor is mixed with 1, 1, 1, 3 , the molar ratio of 3-pentachlorobutane is 15:1, the reaction pressure is 1.5MPa, and the catalyst is SbCl 5 . The composition of the organic matter in the lower layer of the phase separator (8) is analyzed by gas chromatography, and the selectivity of HFC-365mfc is 93.3%, and the others are 6.7%.
Embodiment 2
[0028]The temperature of the low temperature reaction zone (1) of the liquid phase fluorination reactor is controlled at 60°C, the temperature of the low temperature reaction zone (1) is controlled at 90°C, and the HF entering the liquid phase fluorination reactor is mixed with 1, 1, 1, 3 , the molar ratio of 3-pentachlorobutane is 15:1, the reaction pressure is 1.0MPa, and the catalyst is SbCl 5 . The composition of the organic matter in the lower layer of the phase separator (8) is analyzed by gas chromatography, and the selectivity of HFC-365mfc is 92.5%, and the others are 7.5%.
Embodiment 3
[0030] The temperature of the low temperature reaction zone (1) of the liquid phase fluorination reactor is controlled at 90°C, the temperature of the low temperature reaction zone (1) is controlled at 110°C, the HF entering the liquid phase fluorination reactor is mixed with 1, 1, 1, 3 , the molar ratio of 3-pentachlorobutane is 6:1, the reaction pressure is 1.2MPa, and the catalyst is SnCl 4 . The composition of the organic matter in the lower layer of the phase separator (8) was analyzed by gas chromatography, and the selectivity of HFC-365mfc was 90.1%, and the others were 9.9%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com