Controllable polymerization method of industrial-grade monomer
A polymerization method and an industrial-grade technology, which are applied in the field of preparing polymethyl methacrylate by atom transfer radical polymerization, can solve the problems of troublesome polymerization of inhibitors and increased costs, and achieve industrial production convenience, low toxicity, and The effect of reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Embodiment one: synthesis polymethyl methacrylate (PMMA) under deoxygenation condition
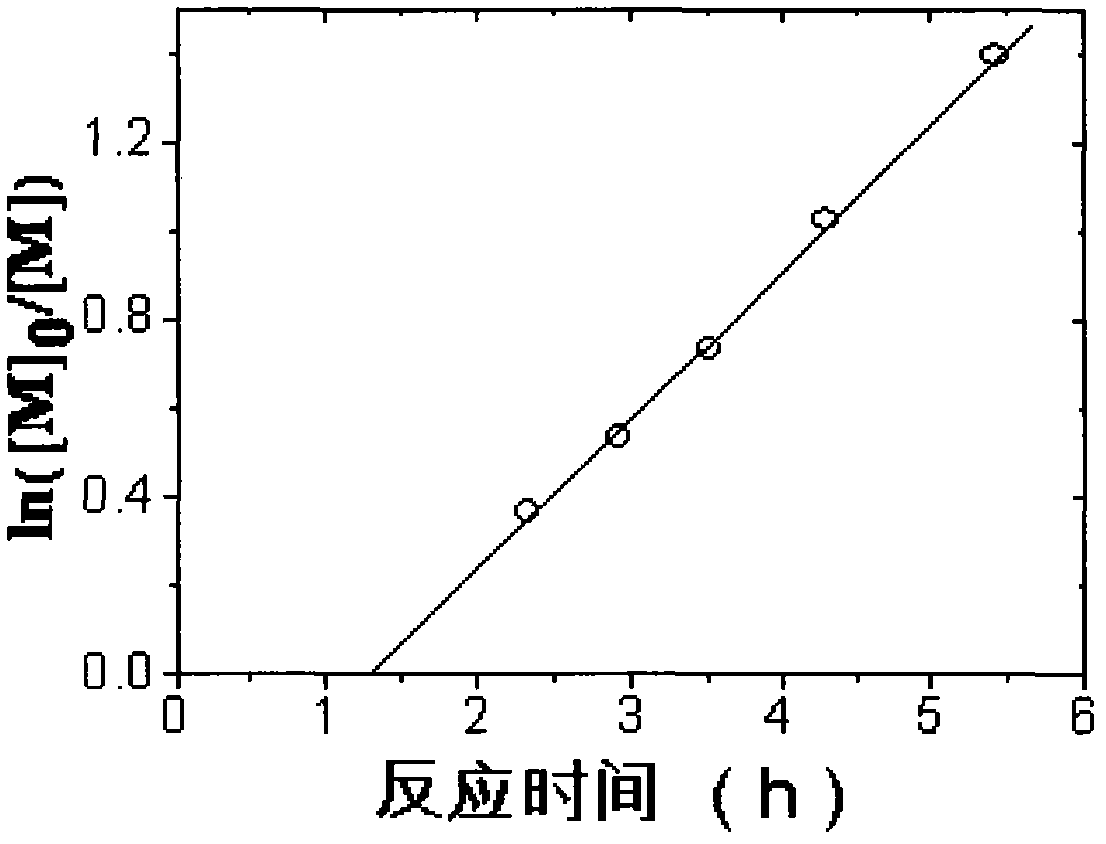
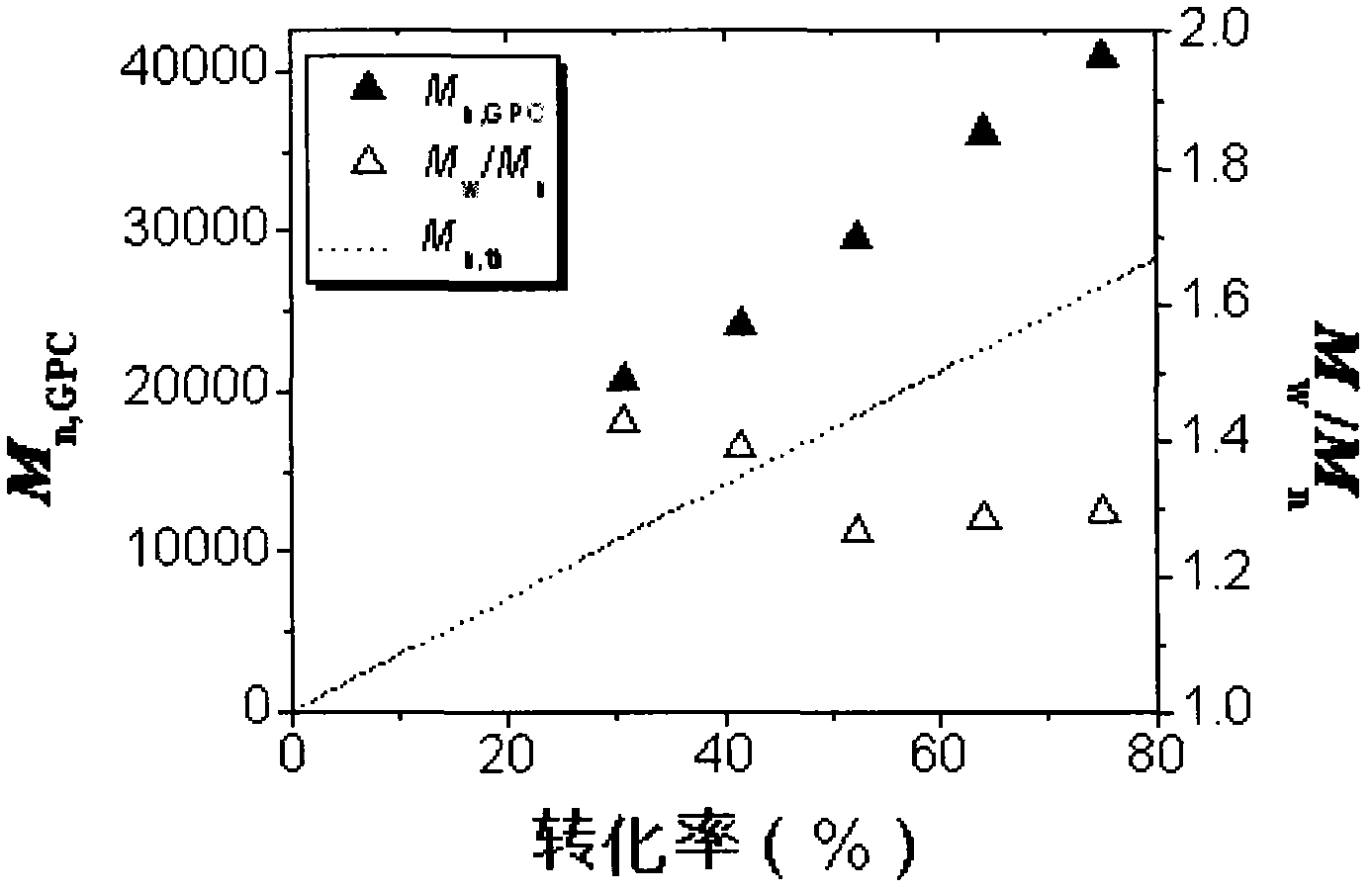
[0028] Add MMA, EBiB, FeCl in a molar ratio of 300:1:0.2:0.6 based on 3mL MMA (unrefined) into several clean 5mL ampoules 3 ·6H 2 O and PPh 3 , add a small stirrer, seal each ampoule via argon gas for 20 minutes, place it in an oil bath at 90°C for reaction, and take samples at certain intervals. Open the seal, dissolve the polymer with a small amount of THF, then pour the solution into a beaker filled with 200mL industrial methanol (with two drops of concentrated hydrochloric acid added in advance) to precipitate, put the beaker in a fume hood and let it stand for more than 10h, and collect the precipitate by suction filtration. Place in a constant temperature oven at 50°C and dry to constant weight, weigh, calculate the conversion rate, and save the product for GPC characterization.
[0029] The result is as figure 1 , figure 2 shown, from figure 1 , we can see that the pol...
Embodiment 2
[0030] Embodiment two: synthesizing PMMA under the presence of air
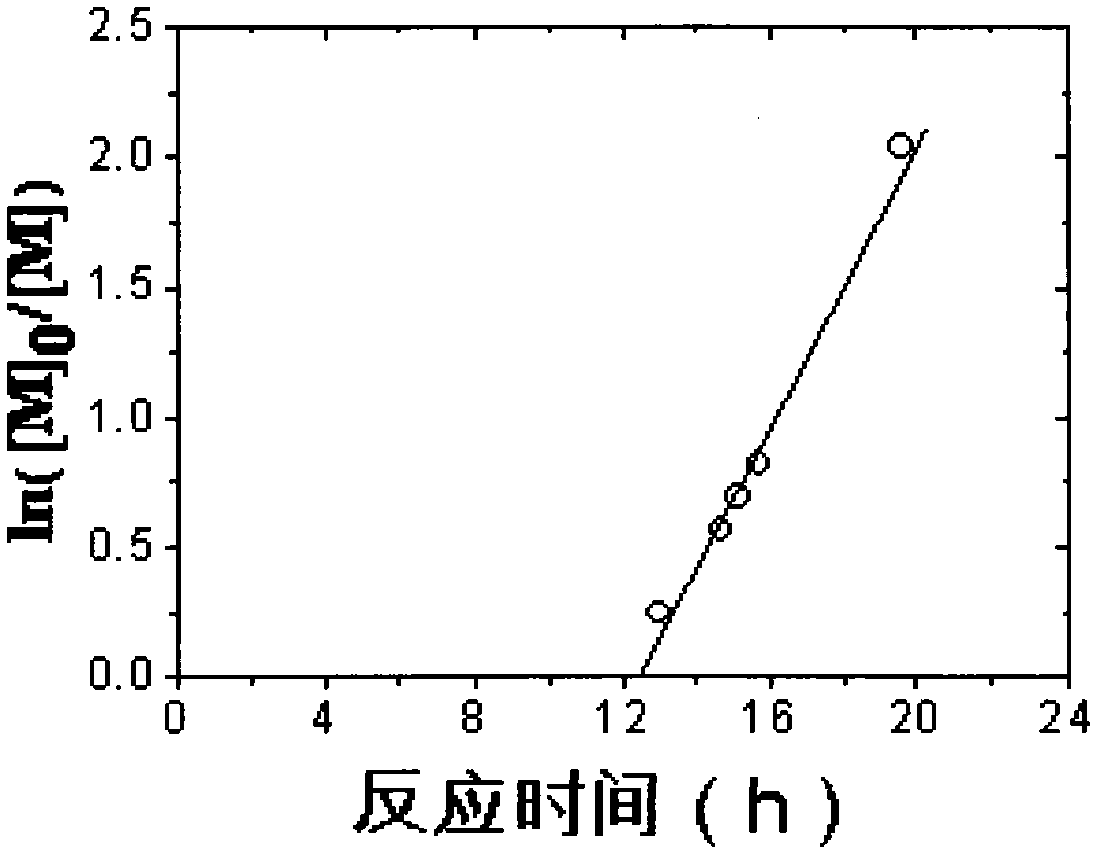
[0031] Add MMA, EBiB, and FeCl in a molar ratio of 300:1:0.2:0.6 based on 3mL MMA (unrefined) into several clean 5mL ampoules 3 ·6H 2 O and PPh 3 , after adding a small stirring bar, seal it directly under air atmosphere (without excluding oxygen), place it in an oil bath at 90°C for reaction, and take samples at certain intervals. Open the seal, dissolve the polymer with a small amount of THF, then pour the solution into a beaker filled with 200mL industrial methanol (with two drops of concentrated hydrochloric acid added in advance) to precipitate, put the beaker in a fume hood and let it stand for more than 10h, and collect the precipitate by suction filtration. Place in a constant temperature oven at 50°C and dry to constant weight, weigh, calculate the conversion rate, and save the product for GPC characterization.
[0032] The result is as image 3 , Figure 4 shown by image 3 It can be seen that...
Embodiment 3
[0033] Example 3: Effect of Catalyst Consumption on Polymerization under the Condition of Deoxygenation
[0034] Add 3mL MMA (unrefined) to 10 clean 5mL ampoules as a basis, different ratios (molar ratios are 300 / 1 / 0.05 / 0.15, 300 / 1 / 0.1 / 0.3, 300 / 1 / 0.2 / 0.6 , 300 / 1 / 0.3 / 0.9, 300 / 1 / 0.5 / 1.5) MMA, EBiB, FeCl 3 ·6H 2 O and PPh 3, two experiments were done for each group of ratios, a small stirring bar was added, each ampoule was sealed with argon gas for 20 minutes, and placed in an oil bath at 90°C for the set time. Open the seal, dissolve the polymer with a small amount of THF, then pour the solution into a beaker containing 200mL industrial methanol (with two drops of concentrated hydrochloric acid added in advance) to precipitate, put the beaker in a fume hood and let it stand for more than 10h, and collect the precipitate by suction filtration. Place in a constant temperature oven at 50°C and dry to constant weight, weigh, calculate the conversion rate, and save the product fo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Bronsted acidity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com