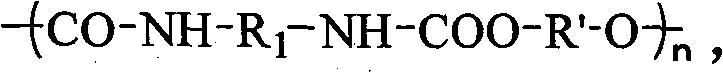Polyacrylate-urethane copolymer and preparation method thereof
A technology of polyacrylate and hydroxyl-terminated polyacrylate, which is applied in the field of chemical materials, can solve the problems of no relevant reports on polyurethane, and achieve the effects of controllable molecular weight, good solubility, and clear structure
Active Publication Date: 2010-12-22
溧阳常大技术转移中心有限公司
View PDF0 Cites 7 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
At present, there are no relevant reports at home and abroad on the research of polyurethane synthesis with hydroxyl-terminated polyacrylate prepared by ATRP method as raw material.
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
Embodiment 2
Embodiment 3
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
| Property | Measurement | Unit |
|---|---|---|
| Glass transition temperature | aaaaa | aaaaa |
| Number average molecular weight | aaaaa | aaaaa |
Login to View More
Abstract
The invention relates to a polyacrylate-urethane copolymer which has a structural formula shown in the specification of the inveniton, wherein n=3-200, carbon atom number of R1 is less than 25, and R' is polyacrylate. A preparation method comprises the steps of: firstly, preparing polyacrylate with one linear or branched end of Br and the other end of hydroxyl, wherein the polyacrylate has molecular weight of 500-10000 and narrower molecular weight distribution, carrying out nucleophilic substitution on terminal bromine of the polyacrylate by N-methyl ethanolamine to obtain hydroxyl terminated polyacrylate; and then reacting the hydroxyl terminated polyacrylate R(HO)n used as raw materials with isocyanates R1(OCN)n in the presence of a given quantity of solvent or in absence of solvent at a certain temperature under the condition of adding a given quantity of catalyst or no catalyst to prepare the novel polyacrylate-urethane copolymer with molecular weight of above 8000 and molecular main chain containing acrylate and urethane structures. The copolymer has better dissolubility, mechanical behavior, adhesive property and thermostabilization, and has respective advantages of the acrylate and the polyurethane.
Description
technical field The invention belongs to the field of chemical materials and relates to the preparation of acrylate-urethane copolymers containing acrylate and urethane structures in the main chain by using hydroxyl-terminated polyacrylate polymers synthesized by atom transfer radical polymerization (ATRP) as raw materials. Background technique Polyurethane has good physical and mechanical properties, excellent cold resistance, elasticity, high gloss, adjustable polymer hardness, and resistance to organic solvents. It is widely used in automobiles, construction, machinery, light industry, medical equipment, and safety protection. and aerospace fields. However, polyurethane resin has disadvantages such as relatively high price and poor aging resistance. Acrylic resin has the advantages of high mechanical strength, aging resistance, light resistance and non-yellowing, and good water resistance, but has disadvantages such as poor resistance to organic solvents, stickiness at h...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More IPC IPC(8): C08G18/62C08F120/16C08F8/26
Inventor 李坚孙治丹范瑞香任强俞强
Owner 溧阳常大技术转移中心有限公司
Features
- Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com