Nucleotide sequence, method and kit for detecting human influenza A virus
An influenza A virus, nucleotide sequence technology, applied in microorganism-based methods, biochemical equipment and methods, and microbial assay/inspection, etc., can solve the problems of false negatives, false positives, low sequence specificity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
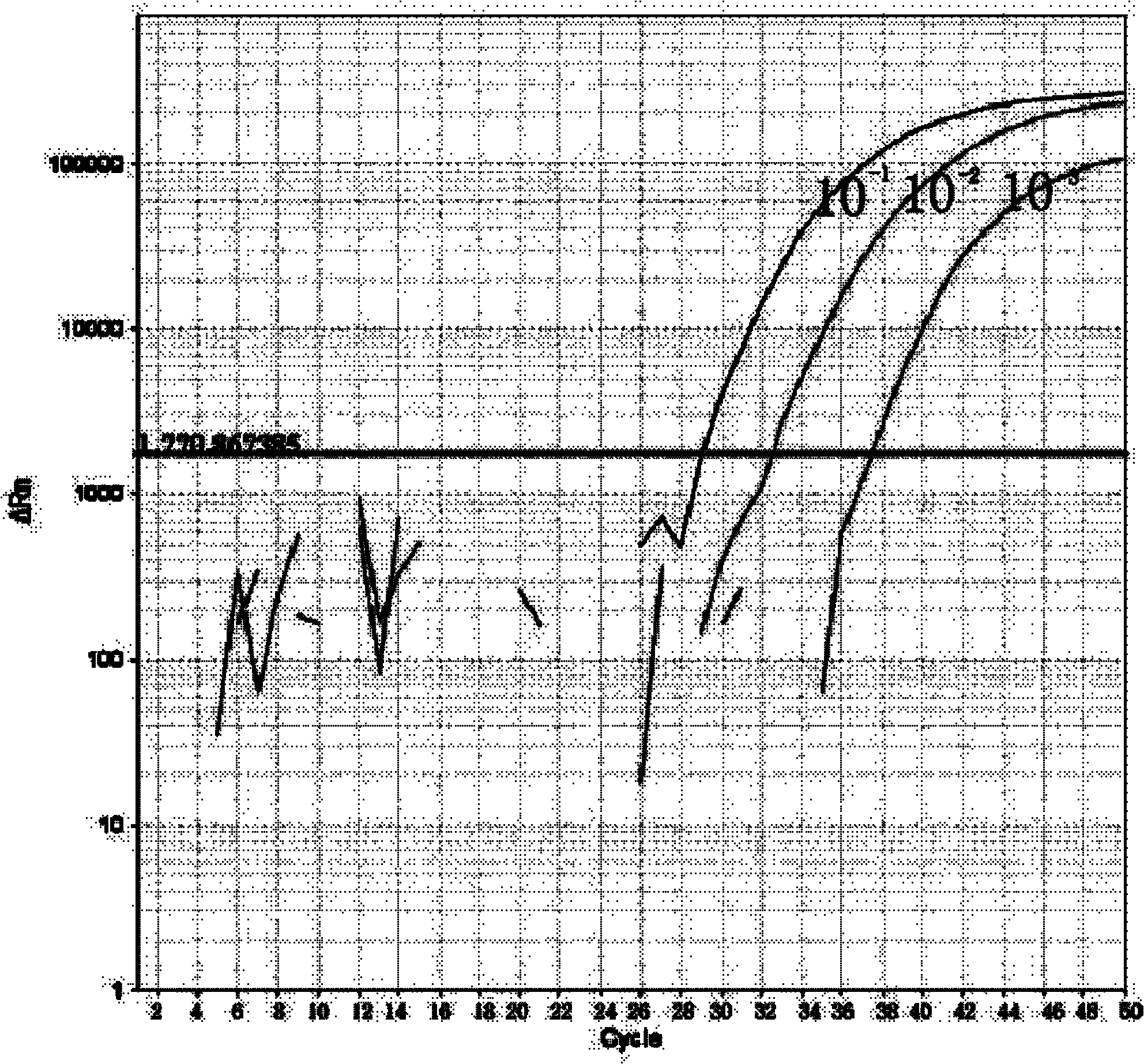
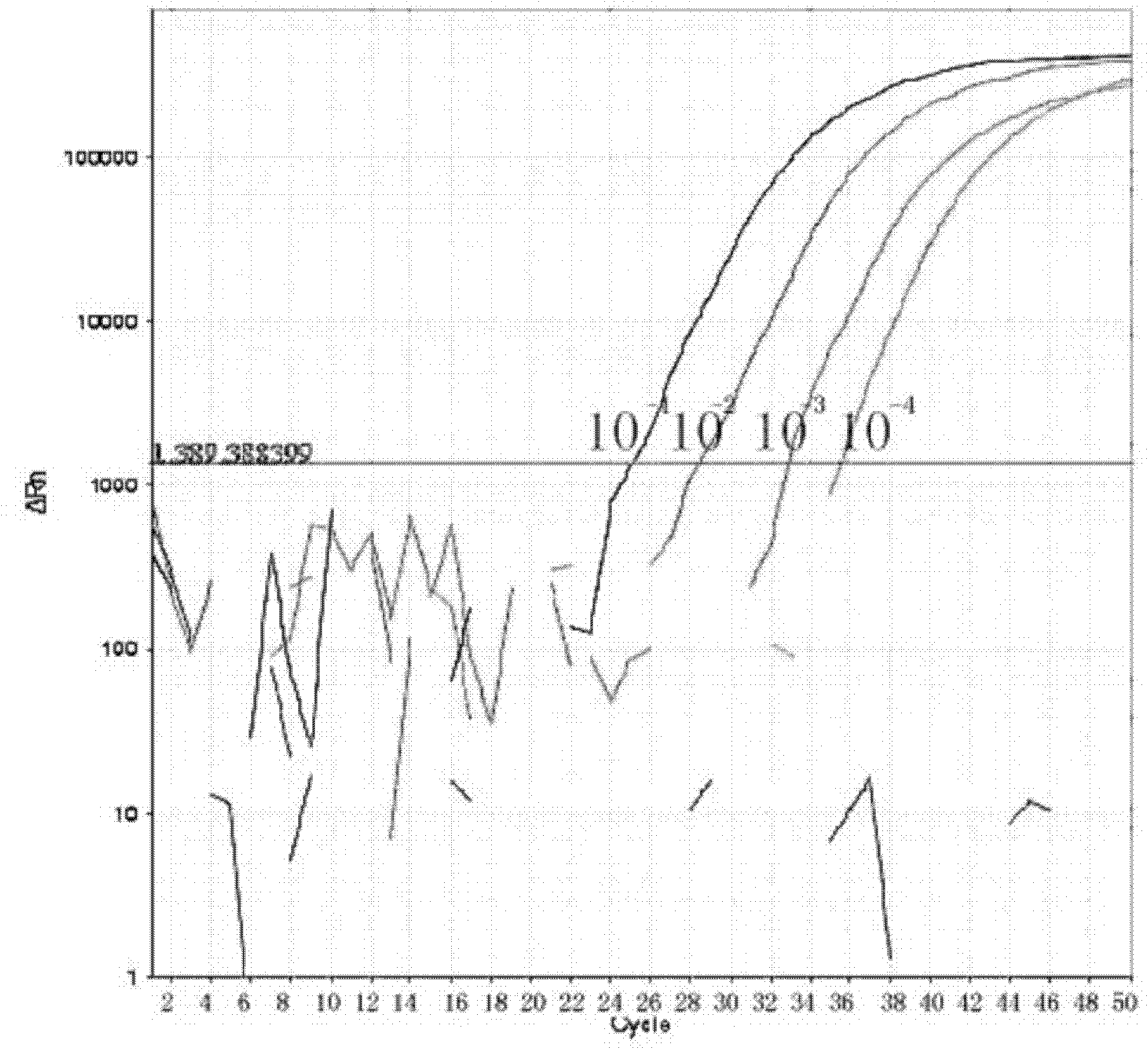
[0063] Example 1 Influenza A virus, seasonal influenza A virus and new type A H1N1 influenza virus typing detection method and kit
[0064] 1: material
[0065] 1.1 Virus samples:
[0066] 1 swine influenza virus sample (virus sample from: Shanghai Veterinary Research Institute, Chinese Academy of Agricultural Sciences);
[0067] 1 sample of the novel influenza A (H1N1) virus that is circulating this time (virus sample from: Queen Mary Hospital, Hong Kong);
[0068] 3 samples of seasonal influenza A virus: 1 seasonal influenza A H1, 1 seasonal influenza A H3, 1 seasonal influenza A H9 (virus samples from: Guangzhou Center for Disease Control and Prevention) ;
[0069] 1 highly pathogenic H5N1 avian influenza virus sample (virus sample from: Hong Kong Queen Mary Hospital);
[0070] 1 copy of influenza B virus (virus sample from: Shanghai Veterinary Research Institute, Chinese Academy of Agricultural Sciences).
[0071] 2. Method
[0072] 2.1 Acquisition of total RNA of th...
Embodiment 2
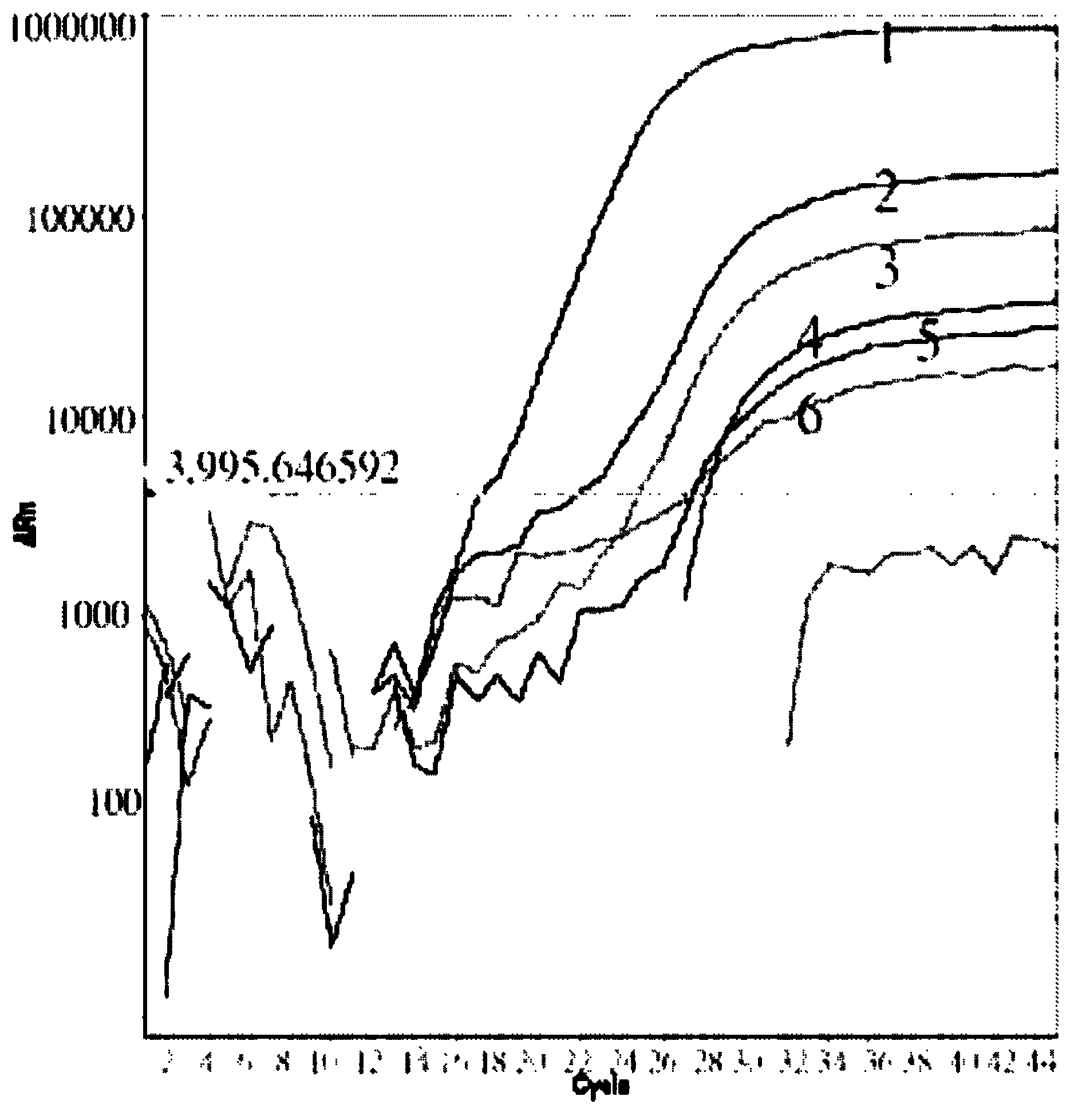
[0090] Example 2 Kit
[0091] The specification of this kit is for 20 people, and the composition of the kit is:
[0092] ingredients
Labeled amount (ml)
ingredients
Labeled amount (ml)
RT-PCR reaction solution
800
New Type A H1N1 Quantitative Reference
product (1×10 -4 TCID 50 )
15
mixed enzyme
40
New Type A H1N1 Quantitative Reference
product (1×10 -3 TCID 50 )
15
15
New Type A H1N1 Quantitative Reference
product (1×10 -2 TCID 50 )
15
500
New Type A H1N1 Quantitative Reference
product (1×10 -1 TCID 50 )
15
DEPC water
500
[0093] Among them, the RT-PCR reaction solution: 100 μl of 10× reverse transcription PCR reaction solution, 40 μl of 10 mM dNTP, 200 ml of 5× reverse transcription enhancement solution, 10 μl of RNase inhibitor, 20 μl of 20 μM prim...
Embodiment 3
[0096] The usage method of embodiment 3 kit:
[0097] Reagent preparation
[0098] 1.1 Thaw the reagent completely before opening the tube, mix well and centrifuge briefly
[0099] 1.2 Preparation: Take 43ml of RT PCR reaction solution and 2ml of mixed enzyme for each reaction, multiply by the number of reaction tubes required (it is recommended to add one more reaction volume as appropriate), add to a total sterile centrifuge tube, shake Mix well.
[0100] 1.3 Packing: Add 45ml of the above-mentioned mixed reaction solution into each PCR reaction tube with a micro-sampler, and store at 4°C.
[0101] Note: It is best to prepare the reaction solution after the RNA extraction is completed.
[0102] 2. Sample RNA extraction: Use Qiagen's RNA kit to process the sample, extract and purify the total RNA, dissolve it in 50ul DEPC water, and follow the manufacturer's instructions for the entire operation.
[0103] 3. Adding samples: Add 5ul of the sample and 5ml of negative contro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com