Medicinal composition for treating gout
A composition and drug technology, applied in the field of pharmaceutical preparations, can solve the problems of liver function damage, aggravation of gout attacks and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
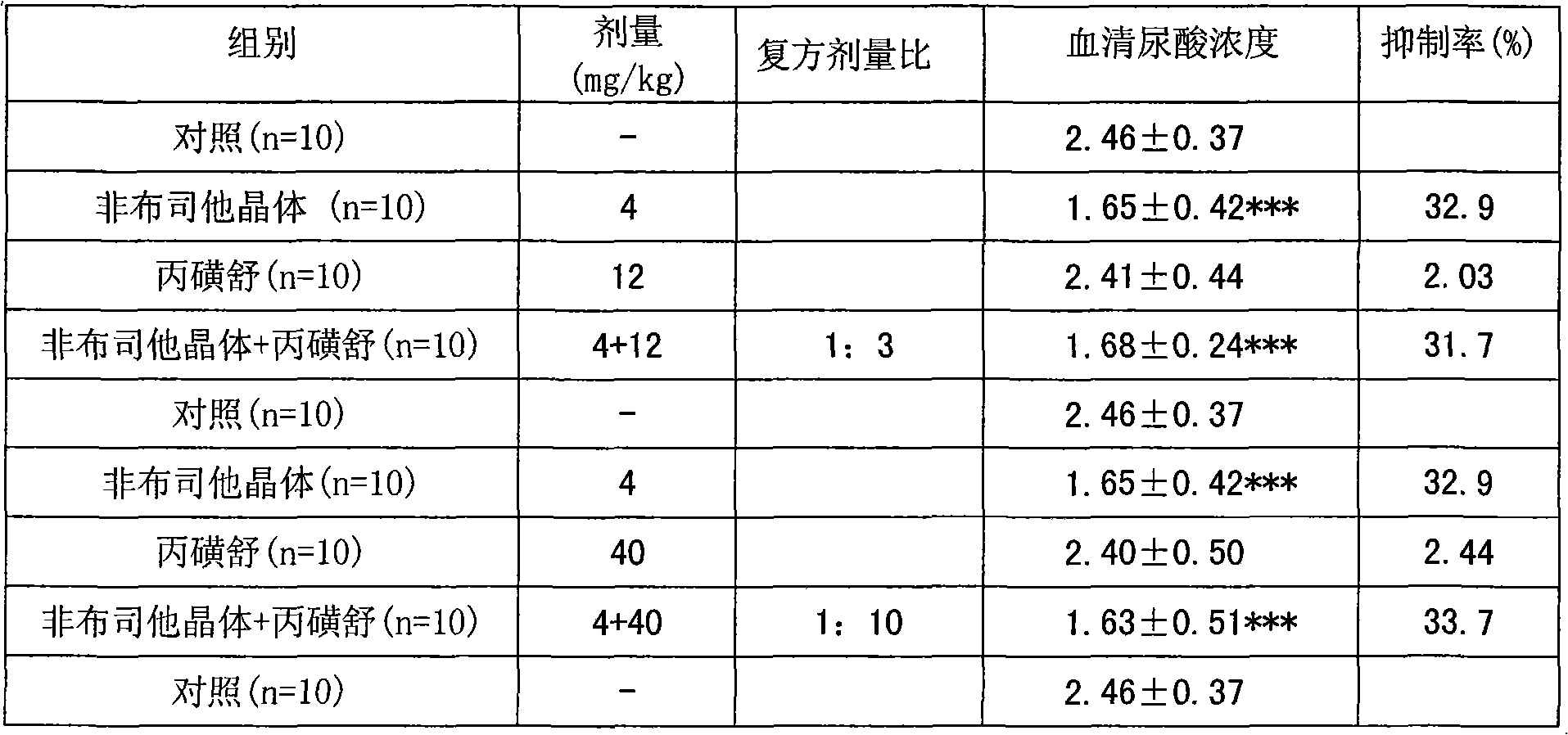
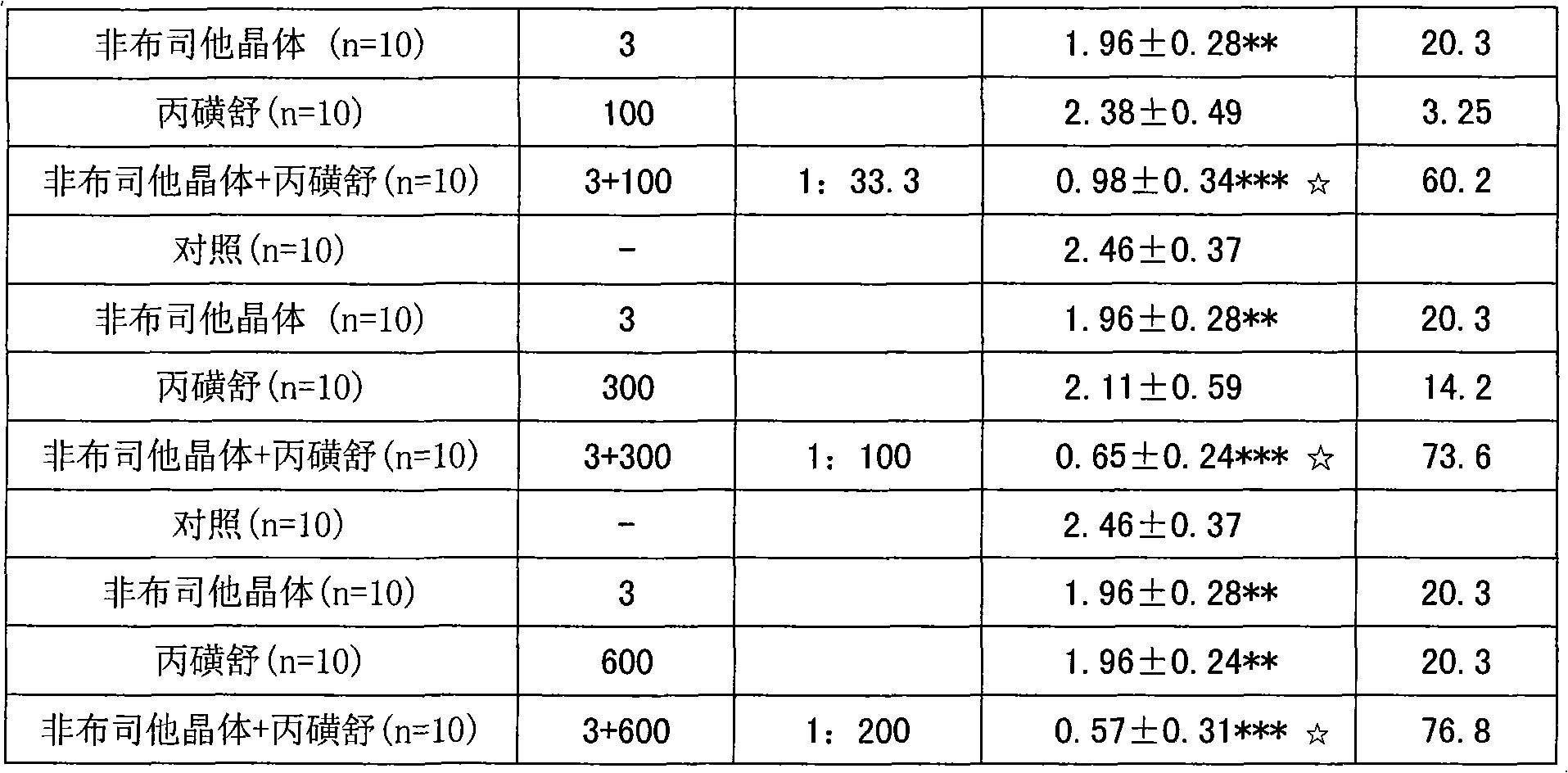
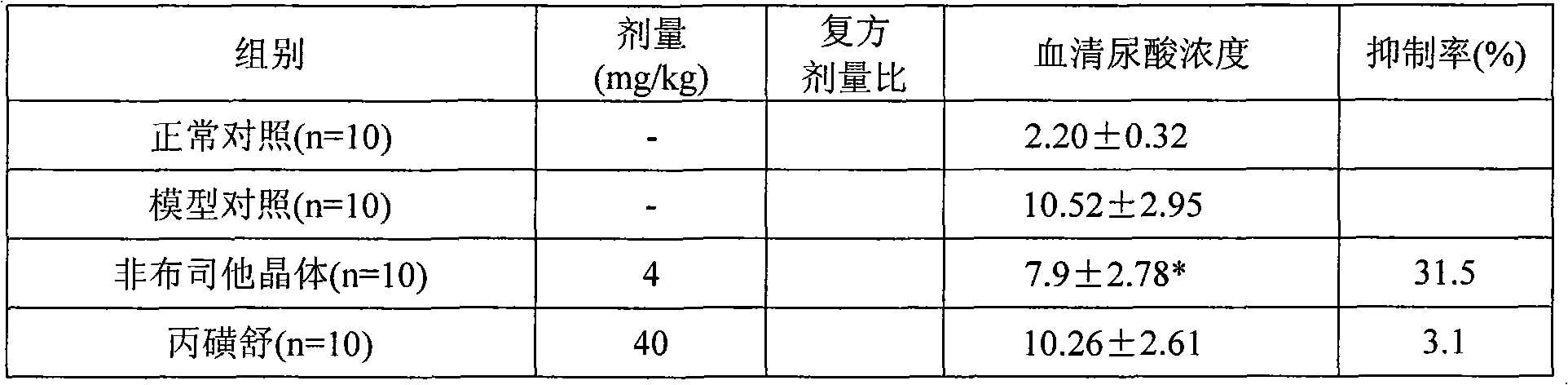
Examples
Embodiment 1
[0053] Preparation of febuxostat crystals:
[0054] Take 2 grams of febuxostat, dissolve it in 60ml of 95% ethanol, cool down, let it stand naturally, precipitate crystals, filter, and dry to obtain 1.94 grams of febuxostat crystals, with a yield of 85%, and then perform SXRD determination. Results: The febuxostat crystal is monoclinic, its space group is P2(1), and the unit cell parameters are: a=4.7179(9) b=17.813(4) c=10.690(2) α=γ=90°, β=99.20(3)°, the unit cell volume is: 886.8(3) For details, please refer to the preparation method disclosed in the application number: 200910068558.2.
Embodiment 2
[0056] Probenecid 1000g, febuxostat crystal 11.5g (equivalent to febuxostat 10g), lactose 675g, microcrystalline cellulose 250g, mix well, add appropriate amount of 10% PVP-ethanol solution to granulate, dry, then add Carboxymethyl starch sodium 83g, magnesium stearate 23.5g, mix well, tablet, film-coated.
Embodiment 3
[0058] Preparation of tablets: mix probenecid 750g, febuxostat crystal 22.9g (equivalent to febuxostat 20g), add lactose 1667g, pregelatinized starch 390g, hydroxypropyl cellulose 15g, cross-linked carboxylate Sodium methylcellulose 63g, magnesium stearate 31.5g, mixed evenly, granulated with pure water, dried, compressed into tablets, coated with film.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com