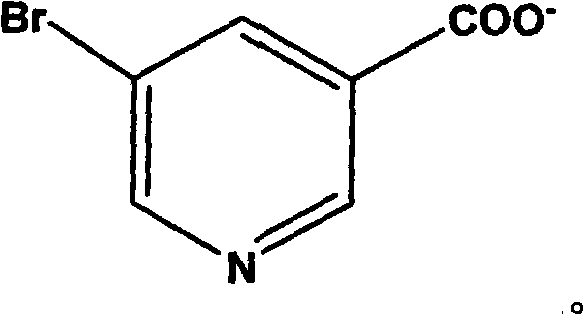
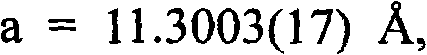Cadmium (II) coordination compound including 5-bromoniacin and preparation method thereof
A complex, nicotinic acid technology, applied in cadmium organic compounds, chemical instruments and methods, organic chemistry and other directions, can solve problems such as unmanned registration, and achieve the effects of simple and easy operation, high yield and good thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1 The preparation process of the cadmium (II) complex containing 5-bromonicotinic acid is as follows:
[0030] 0.1mmol Cd(NO 3 ) 2 4H 2 O is placed at the bottom of the test tube and dissolved with 5 mL of distilled water; 15 mL of a mixture of methanol and water at a volume ratio of 1:1 is added in the middle; 0.05 mmol HL is dissolved with 5 mL of methanol and placed on the upper layer of the test tube; then sealed and left for 20 days, the solution on the wall of the test tube Colorless blocky single crystal P2 appears at the junction of the interface 1 / c, the crystal is washed with distilled water and ethanol in sequence, and dried in vacuum to obtain the target product with a yield of about 75%.
[0031] The main infrared absorption peaks of the target product are: 1601vs, 1554vs, 1428w, 1385vs, 1294m, 1234w, 1188w, 1128m, 1094m, 1027w, 909w, 879m, 783s, 738s, 690s, 618w, 569w, 463w; the elemental analysis results For: theoretical value: H, 1.18; C, 28...
Embodiment 2
[0032] Example 2 The preparation process of the cadmium (II) complex containing 5-bromonicotinic acid is as follows:
[0033] 0.1mmol CdSO 4 Put it at the bottom of the test tube and dissolve it with 5 mL of distilled water; add 16 mL of a mixture of methanol and water with a volume ratio of 1:1 in the middle; dissolve 0.05 mmol HL with 5 mL of methanol and place it on the upper layer of the test tube; A colorless blocky single crystal appeared at the junction of , and the crystal was washed with distilled water and ethanol in sequence, and dried in vacuum to obtain the target product with a yield of about 72%.
[0034] The main infrared absorption peaks of the target product are: 1601vs, 1554vs, 1428w, 1385vs, 1294m, 1234w, 1188w, 1128m, 1094m, 1027w, 909w, 879m, 783s, 738s, 690s, 618w, 569w, 463w; the elemental analysis results For: theoretical value: H, 1.18; C, 28.02; N 5.45%, experimental value: H, 1.10; C, 28.17; N, 5.51%.
Embodiment 3
[0035] Example 3 The preparation process of the cadmium (II) complex containing 5-bromonicotinic acid is as follows:
[0036] 0.12mmol Cd(ClO 4 ) 2 ·6H 2 O is placed at the bottom of the test tube and dissolved with 4 mL of distilled water; 20 mL of a mixture of methanol and water with a volume ratio of 1:1.3 is added in the middle; 0.05 mmol HL is dissolved with 4 mL of methanol and placed on the upper layer of the test tube; then sealed and left for 25 days, the solution on the wall of the test tube A colorless blocky single crystal appeared at the junction of the interface, and the crystal was washed with distilled water and ethanol in sequence, and dried in vacuum to obtain the target product with a yield of about 70%.
[0037]The main infrared absorption peaks of the target product are: 1601vs, 1554vs, 1428w, 1385vs, 1294m, 1234w, 1188w, 1128m, 1094m, 1027w, 909w, 879m, 783s, 738s, 690s, 618w, 569w, 463w; the elemental analysis results For: theoretical value: H, 1.18; ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com