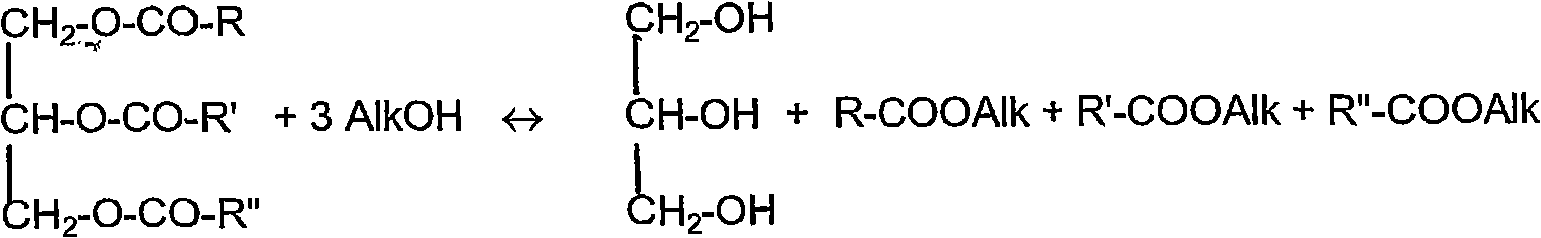Transesterification of vegetable oils
A technology for transesterification and refining vegetable oil, which is applied in the treatment of hydrocarbon oil, biofuel, petroleum industry, etc., can solve the problem of not disclosing specific methods for refining vegetable oil, etc., and achieves the effect of multiple economic benefits
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0077] Pretreatment: 250 g of partially refined sunflower oil was mixed with 75 g of n-hexane and placed in a Parr type reactor. 2.5 g of commercially available Amberlist 15 ion exchange resin and 10 g of methanol were added to the system. The reactor was pressurized to 15 bar with nitrogen. The whole system was placed at 80°C and stirred continuously for 30 minutes. Upon completion, the reactor was rapidly cooled to room temperature with a stream of tap water and depressurized.
[0078] Repeat the above process to obtain analytical samples after washing and evaporation.
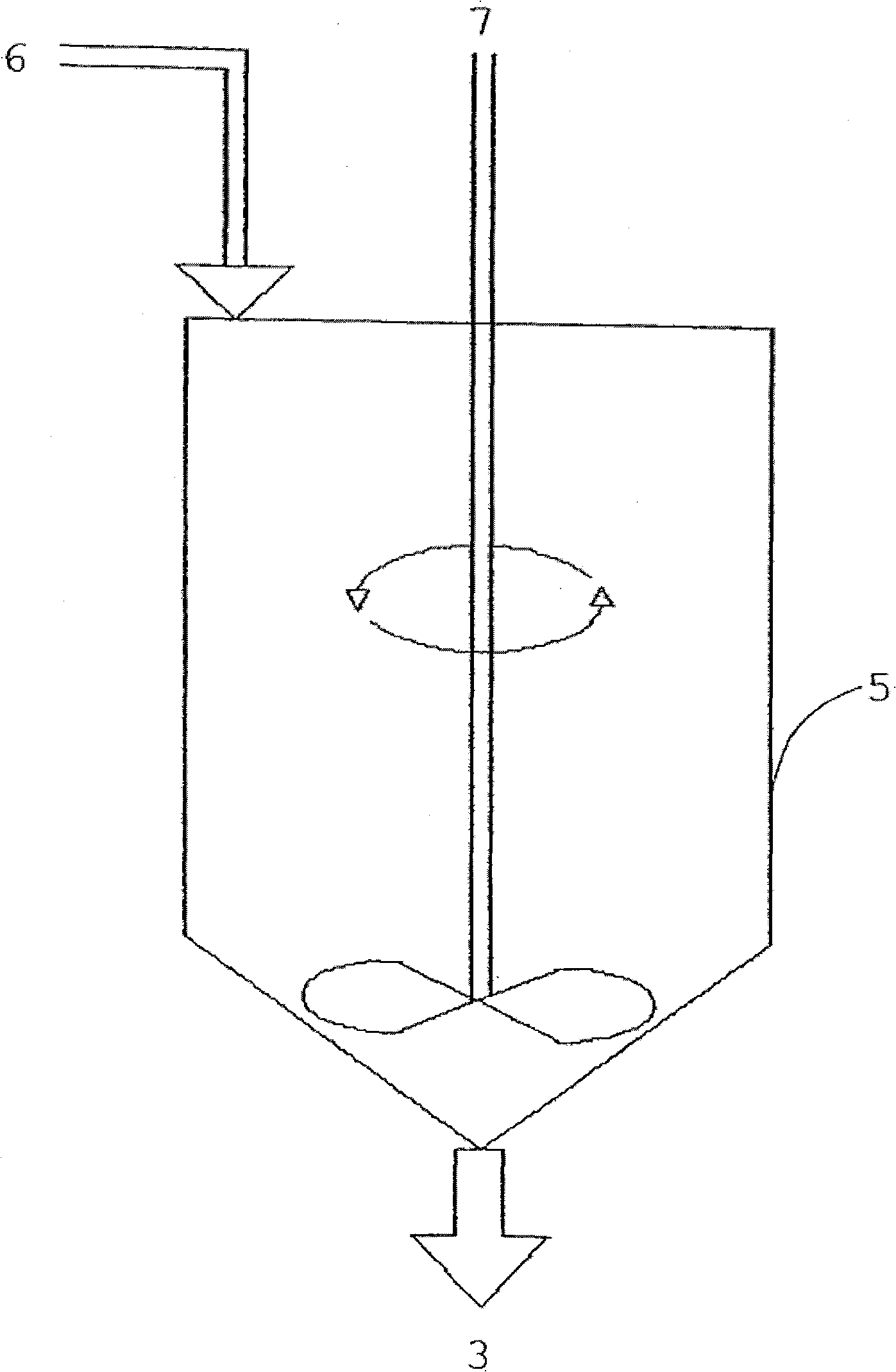
[0079]Transesterification: The contents of the reactor were transferred to a 4-neck flask of 1 liter capacity through which was connected a reflux cooler, funnel drop gauge, thermometer and a stirrer on top through the connection of the flask. A few pieces of zeolite and 20 g of methanol (with 15% KOH content) were added to the system. The entire contents were brought to boiling conditions (t = 59°C) and...
Embodiment 2
[0085] Pretreatment: 250 g of partially refined sunflower oil was mixed with 75 g of n-hexane and placed in a Parr type reactor. 2.5 g of commercially available Amberlist 15 ion exchange resin and 10 g of methanol were added to the system. The reactor was pressurized to 15 bar with nitrogen. The whole system was placed at 80°C and stirred continuously for 30 minutes. After completion, the reactor was rapidly cooled to room temperature with a stream of tap water and depressurized.
[0086] Transesterification: The contents of the reactor were transferred to a 4-neck flask of 1 liter capacity through which a reflux cooler, a funnel drop gauge, a thermometer and an overhead stirrer were connected through the connection. A few pieces of zeolite and 20 g of methanol (with 15% KOH content) were added to the system. The entire contents were brought to boiling conditions (t = 59°C) and stirring was continued at this temperature for 10 minutes, then stirring was stopped and the reac...
Embodiment 3
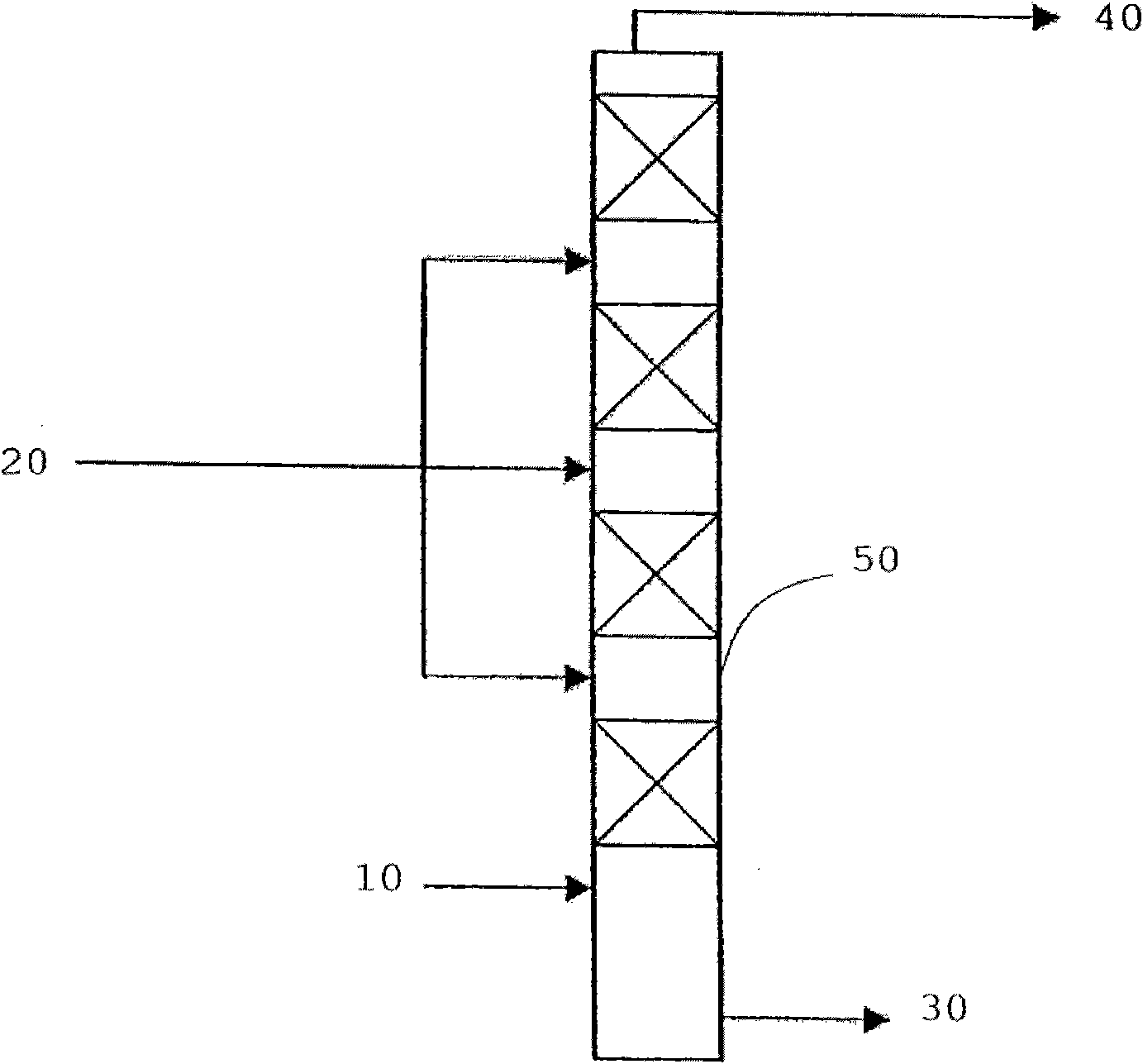
[0093] Pretreatment: Partially refined sunflower oil is mixed with n-hexane in a 4:1 [g:g] ratio and transferred to a reverse extractor where it is contacted with a solvent in a ratio of 1:10 [g:g] 10% KOH aqueous solution and N-methyl-2-pyrrolidone (BASF). The extraction process was carried out at room temperature.
[0094] Transesterification: 400 g of the pretreated raffinate was transferred to a 1 liter capacity 4-neck flask through which was connected a reflux cooler, funnel drop gauge, thermometer and a stirrer on top through the connection of the flask. A few pieces of zeolite and 35 g of methanol (with 15% KOH content) were added to the system. The entire contents were brought to boiling conditions (t = 59°C) and stirring was continued at this temperature for 10 minutes, then stirring was stopped and the reaction mixture was allowed to stand for an additional 10 minutes.
[0095] Refining: Cool the above system to 40°C, transfer the reaction mixture to a separatory f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com