Catalyst, method for producing the same, and use of the same
A manufacturing method and catalyst technology, applied in the direction of catalyst activation/preparation, chemical instruments and methods, physical/chemical process catalysts, etc., can solve problems such as carbon and nitrogen oxides that have not been discussed, and achieve the effect of low-cost performance and high oxygen reduction ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0135] 1. Preparation of Catalyst
[0136] Titanium carbide (TiC) 5.10g (85mmol), titanium oxide (TiO 2 ) 0.80 g (10 mmol) and 0.31 g (5 mmol) of titanium nitride (TiN) were well mixed, and heated at 1800° C. in a nitrogen atmosphere for 3 hours to obtain 5.73 g of titanium carbonitride. Since it is a sintered body, the obtained titanium carbonitride was pulverized with an automatic mortar.
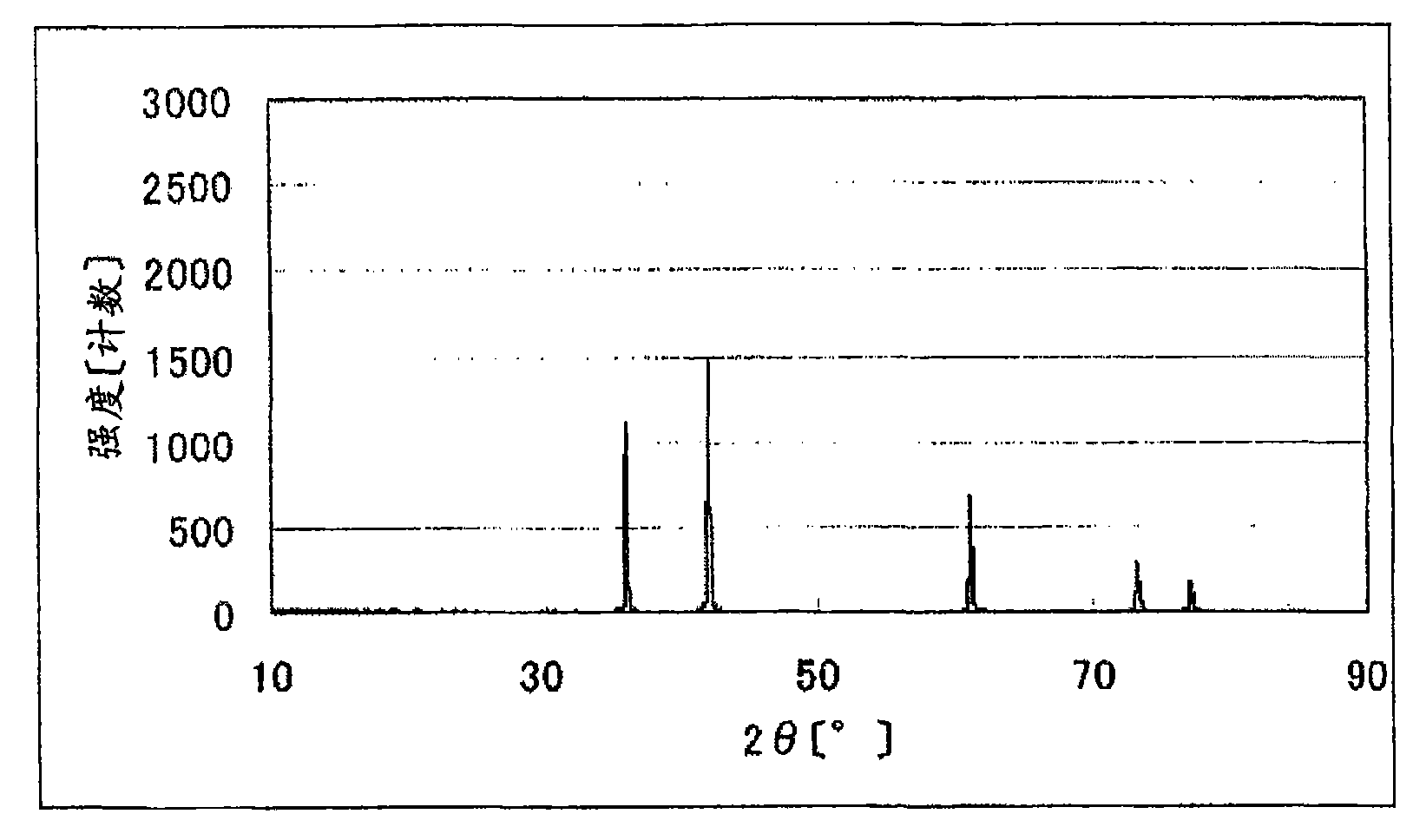
[0137] The powder X-ray diffraction spectrum of the obtained titanium carbonitride is shown in figure 1 .
[0138] In addition, Table 1 shows the elemental analysis results of the obtained titanium carbonitride.
[0139] While flowing nitrogen gas containing 1% by volume of oxygen and 4% by volume of hydrogen, 298 mg of the obtained titanium carbonitride was heated in a tubular furnace at 1000° C. for 10 hours to obtain titanium carbonitride (hereinafter also referred to as "Catalyst (1)".) 393mg.
[0140] The powder X-ray diffraction spectrum of the catalyst (1) obtained is shown in...
Embodiment 2
[0153] 1. Preparation of Catalyst
[0154] While flowing nitrogen gas containing 1.5% by volume of oxygen and 4% by volume of hydrogen, 314 mg of titanium carbonitride obtained in Example 1 was heated in a tubular furnace at 1000° C. for 3 hours, thereby obtaining titanium carbonitride (Hereafter also referred to as "catalyst (2)".) 411 mg.
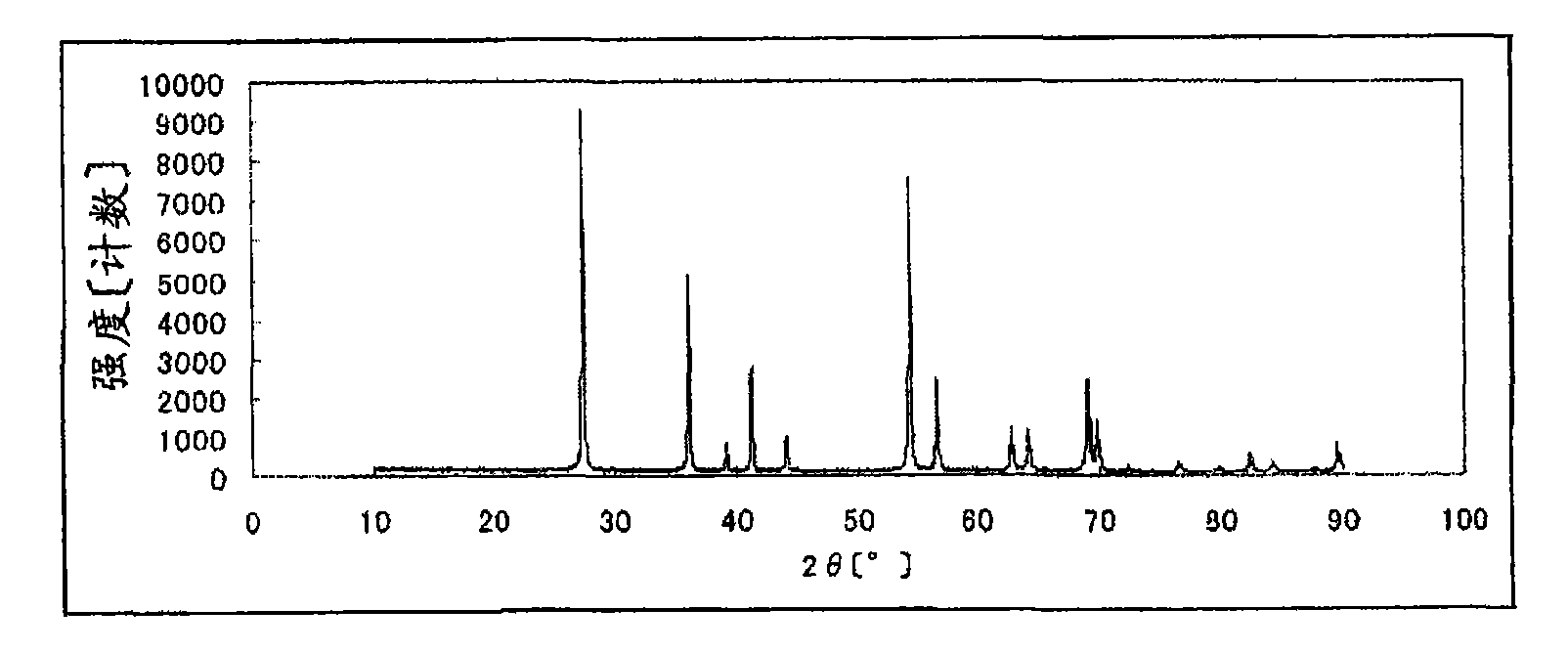
[0155]The powder X-ray diffraction spectrum of the catalyst (2) obtained is shown in Figure 4 .
[0156] In addition, the elemental analysis results of the catalyst (2) are shown in Table 1.
[0157] 2. Manufacture of electrodes for fuel cells
[0158] Except having used the above-mentioned catalyst (2), it carried out similarly to Example 1, and obtained the electrode (2) for fuel cells.
[0159] 3. Evaluation of Oxygen Reduction Capacity
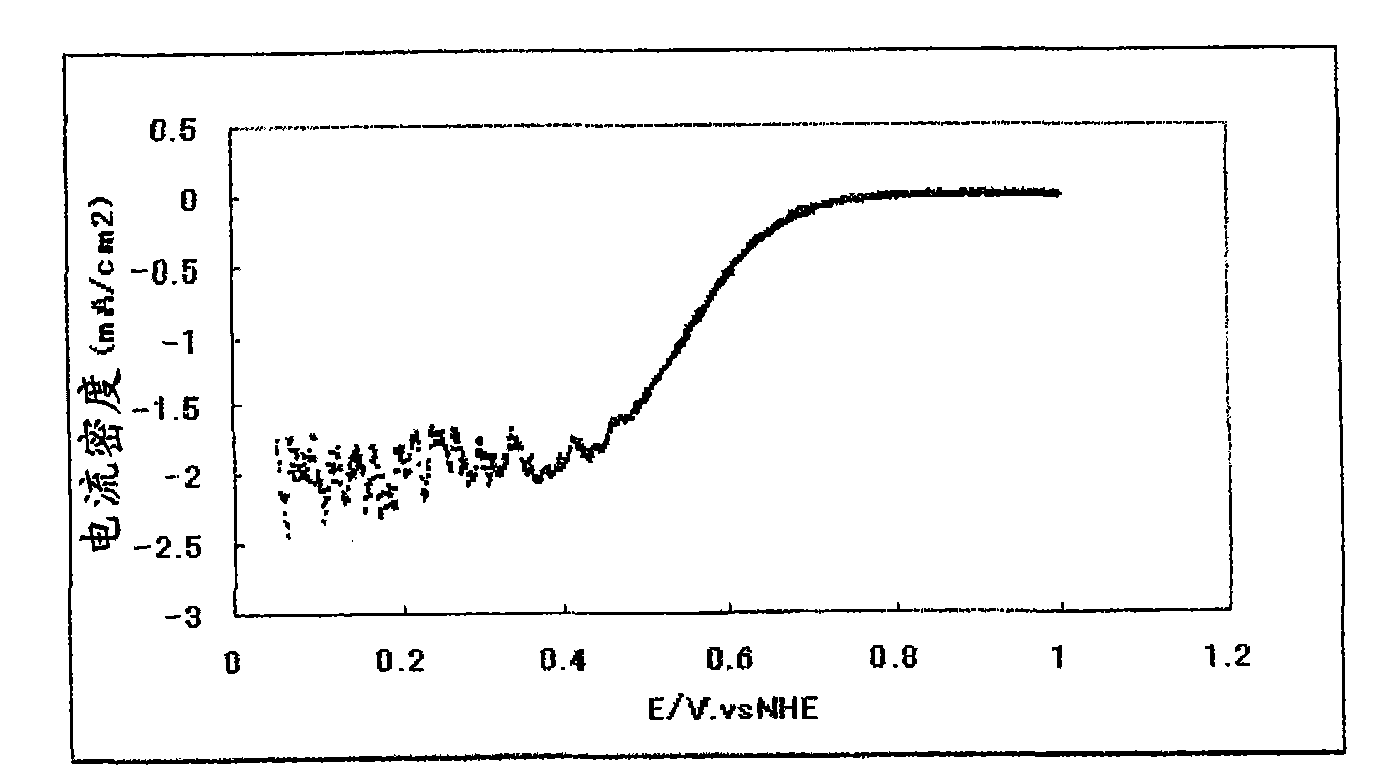
[0160] The catalytic ability (oxygen reducing ability) was evaluated in the same manner as in Example 1, except that the fuel cell electrode (2) described above was used.
[0161] Figure 5 Th...
Embodiment 3
[0164] 1. Preparation of Catalyst
[0165] While flowing nitrogen gas containing 1.0% by volume of oxygen and 1.3% by volume of hydrogen, 314 mg of titanium carbonitride obtained in Example 1 was heated in a tubular furnace at 1000° C. for 3 hours, thereby obtaining titanium carbonitride (Hereafter also referred to as "catalyst (3)".) 415 mg.
[0166] The powder X-ray diffraction spectrum of the catalyst (3) obtained is shown in Figure 7 .
[0167] In addition, the elemental analysis results of the catalyst (3) are shown in Table 1.
[0168] 2. Manufacture of electrodes for fuel cells
[0169] Except having used the above-mentioned catalyst (3), it carried out similarly to Example 1, and obtained the electrode (3) for fuel cells.
[0170] 3. Evaluation of Oxygen Reduction Capacity
[0171] The catalytic ability (oxygen reducing ability) was evaluated in the same manner as in Example 1, except that the fuel cell electrode (3) described above was used.
[0172] Figure 6...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com