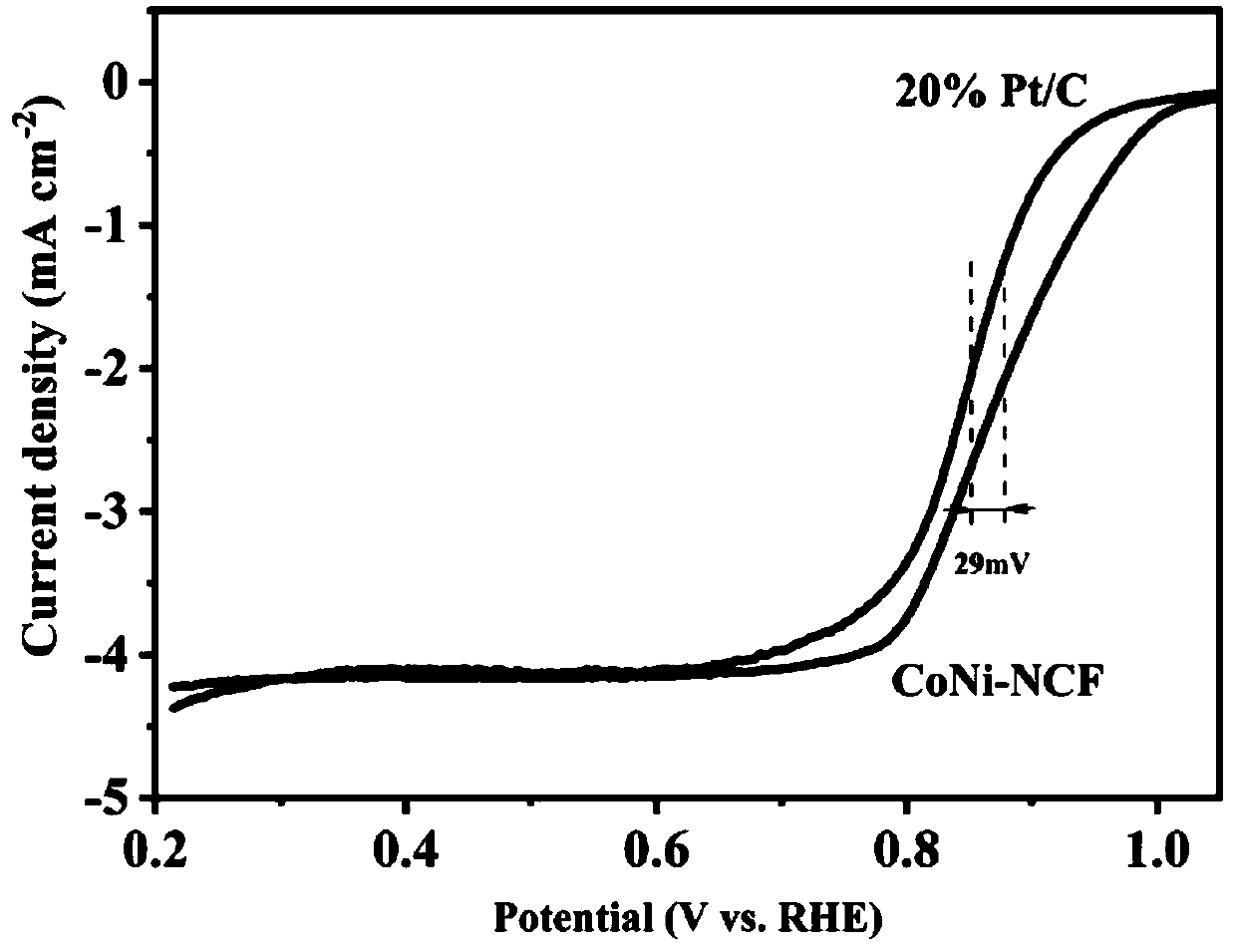
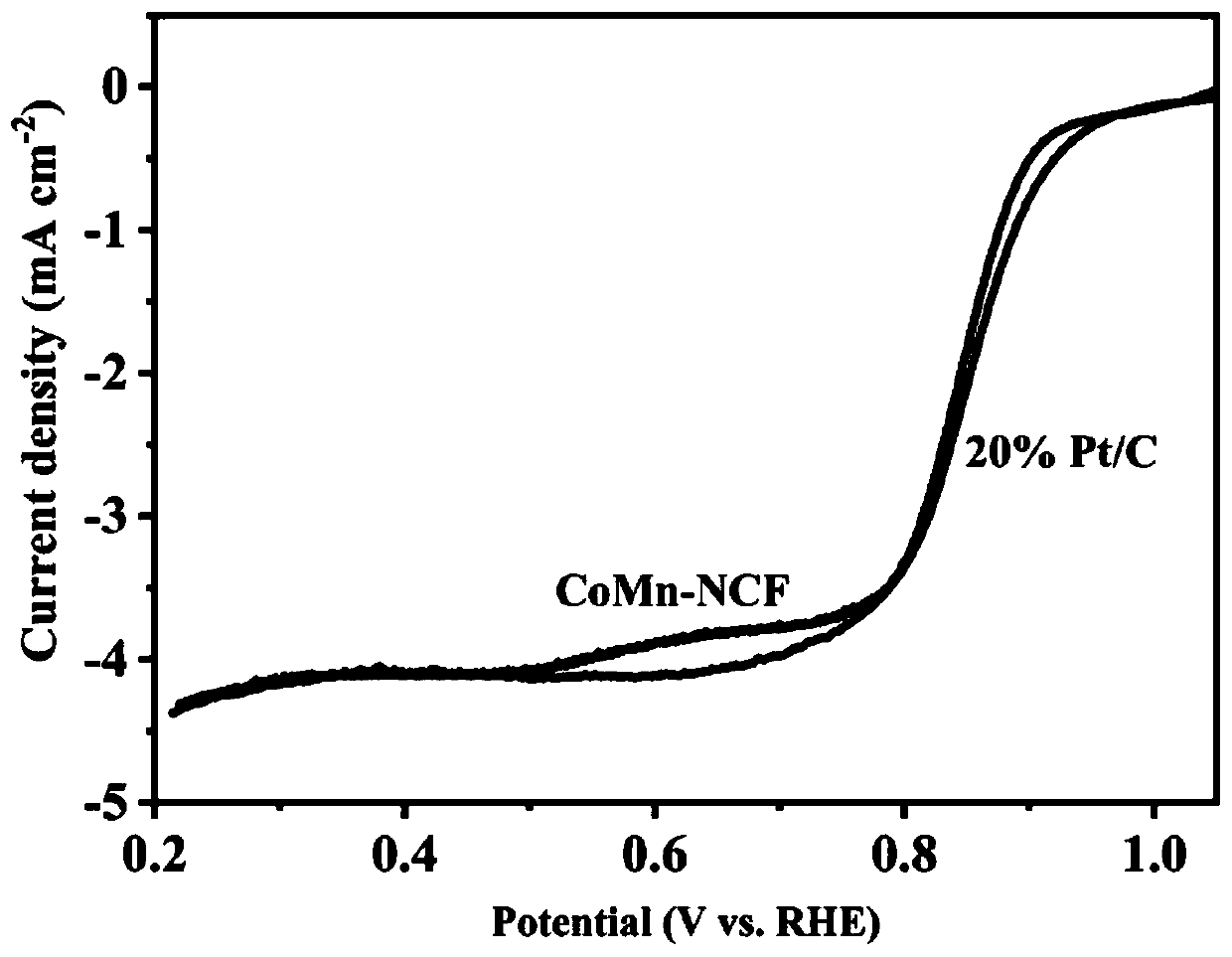
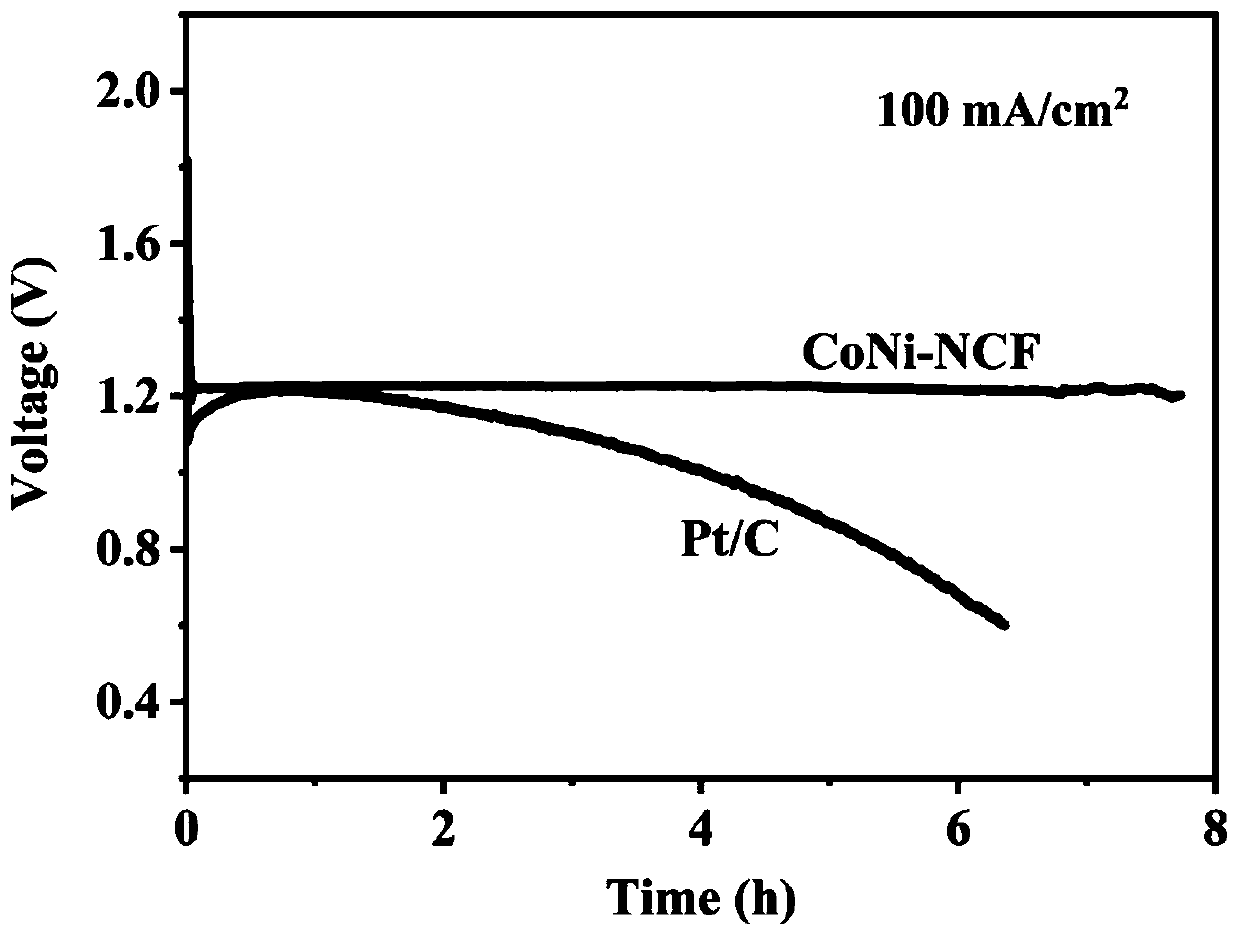
Nitrogen-doped porous carbon-coated non-noble metal alloy composite oxygen reduction catalyst and preparation method thereof
A nitrogen-doped carbon and catalyst technology, applied in electrical components, battery electrodes, fuel cell-type half-cells, and primary-cell half-cells, etc. problem, to achieve the effect of simple method, excellent oxygen reduction performance, and strong operability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] This implementation includes the following steps:
[0033] 1) Weigh 2.70g of dimethylimidazole and add it into 50mL of deionized water, stir for 20min, and configure solution A. Weigh 0.75mL tetraethyl orthosilicate solution, 0.30g cobalt nitrate hexahydrate, 0.0275g nickel nitrate hexahydrate and dissolve in 25mL deionized water, and weigh to form solution B. Slowly add solution B to solution A dropwise, continue to stir for more than 3 hours, centrifuge and wash the suspension several times, then dry it in an oven at 80°C for 48 hours, and cool to room temperature to obtain the precursor of the reactant.
[0034] 2) Grind the reactant precursor in step 1) into a powder, put the powder in a quartz boat, raise the temperature to 900°C at a rate of 5°C / min under a nitrogen atmosphere, and heat-treat at this temperature for 2 hours , cooled to room temperature.
[0035] 3) Dissolve the powder in 4mol / L KOH solution, wash with deionized water and absolute ethanol after 6...
Embodiment 2
[0038] This implementation includes the following steps:
[0039] 1) Weigh 3.60g of dimethylimidazole and add it into 50mL of deionized water, stir for 20min, and configure solution A. Weigh 1.0 mL of tetraethyl orthosilicate solution, 0.30 g of cobalt nitrate hexahydrate, and 0.050 g of manganese acetate tetrahydrate, dissolve them in 25 mL of deionized water, and weigh to form solution B. Slowly add solution B to solution A dropwise, continue to stir for more than 4 hours, centrifuge and wash the suspension several times, then dry it in an oven at 80°C for 24 hours, and cool to room temperature to obtain the precursor of the reactant.
[0040] 2) Grind the reactant precursor in step 1) into a powder, place the powder in a quartz boat, raise the temperature to 800°C at a rate of 10°C / min under a nitrogen atmosphere, and heat-treat at this temperature for 2 hours , cooled to room temperature.
[0041] 3) Dissolve the powder in 6mol / L KOH solution, wash with deionized water a...
Embodiment 3
[0044] This implementation includes the following steps:
[0045] 1) The acetylene black of 6 weight parts, the catalyst prepared in the embodiment 1 of 2 weight parts, the polytetrafluoroethylene emulsion (60wt%) of 2 weight parts are used for preparing catalytic layer, take ethanol as solvent respectively to solid Stir the powder evenly, then add the weighed polytetrafluoroethylene emulsion, stir and disperse evenly, and then roll and shape repeatedly under a roller press to prepare a catalytic membrane with a thickness of 1.0mm.
[0046] 2) Take by weighing 5 parts by weight of acetylene black, 1 part by weight of pore-forming agent, and 4 parts by weight of polytetrafluoroethylene emulsion (60wt%) for the preparation of a waterproof layer, using ethanol as a solvent to stir the solid powder evenly, and then Add the weighed polytetrafluoroethylene emulsion, stir and disperse evenly, and then repeatedly roll and form under the roller press to prepare a waterproof and breathabl...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com