Preparation method of lithium iron phosphate
A technology of lithium iron phosphate and lithium salt, which is applied in the field of preparation of lithium iron phosphate materials, can solve the problems of poor high-rate charge and discharge performance, difficulties in practical application of materials, and large grain size of products, and achieve high-rate charge and discharge performance Good, large specific capacity, high conductivity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Dissolve 42 grams of HEDP in 100 grams of water, stir well until completely dissolved, add 80.8 grams of ferric nitrate, 1.8 grams of glucose and 8.4 grams of lithium hydroxide to mix, and at the same time heat and stir in a water bath at 100°C for 2 hours to remove water, namely Obtain lithium iron phosphate precursor. Move the precursor into a high-temperature atmosphere furnace, heat up to 400°C at a rate of 5°C / min in an atmosphere of argon, nitrogen or nitrogen-hydrogen mixture, roast at a constant temperature for 4 hours, and then raise the temperature to 600°C at the same rate, Calcined at a constant temperature for 10 hours and then cooled naturally to room temperature to obtain lithium iron phosphate, a positive electrode material for a lithium ion battery.
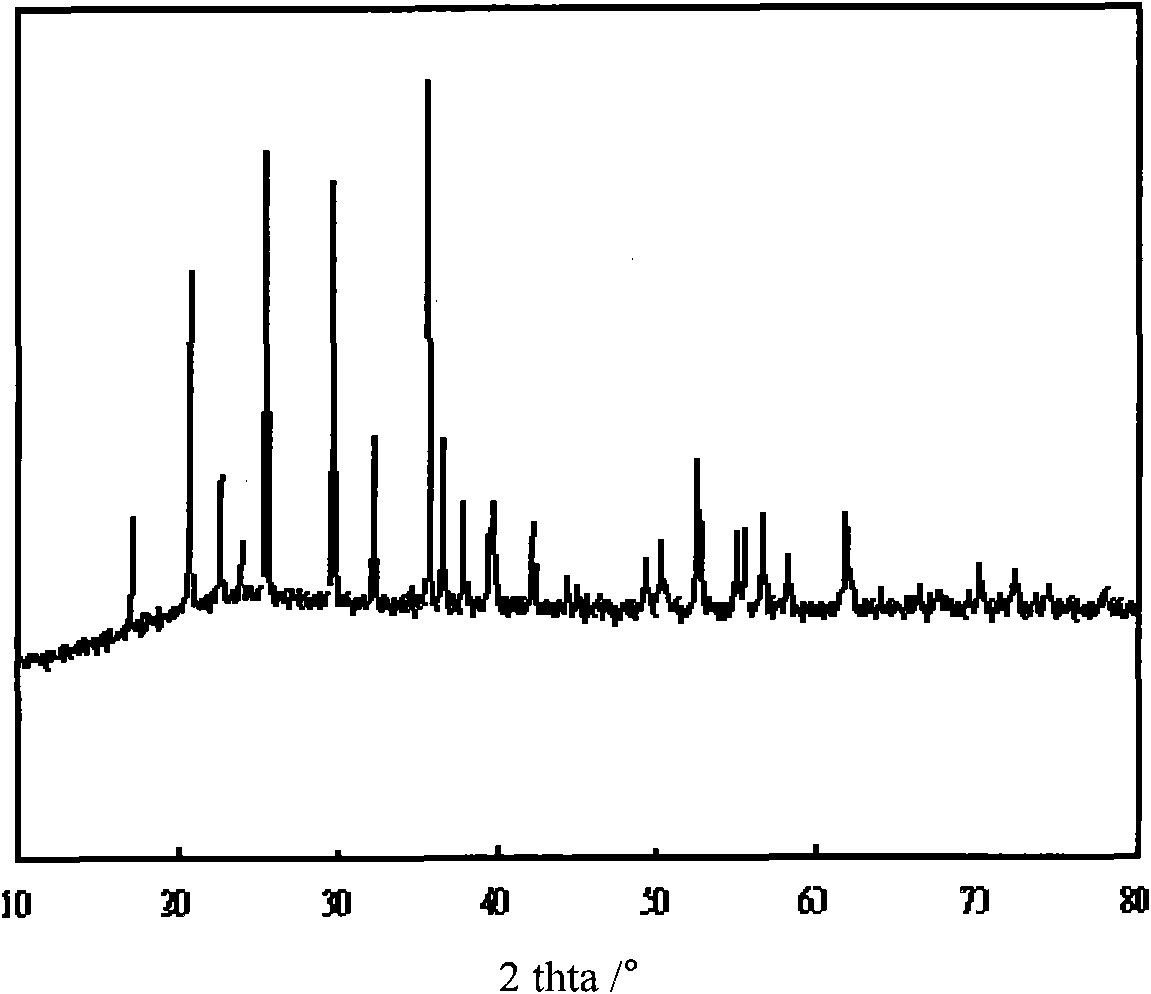
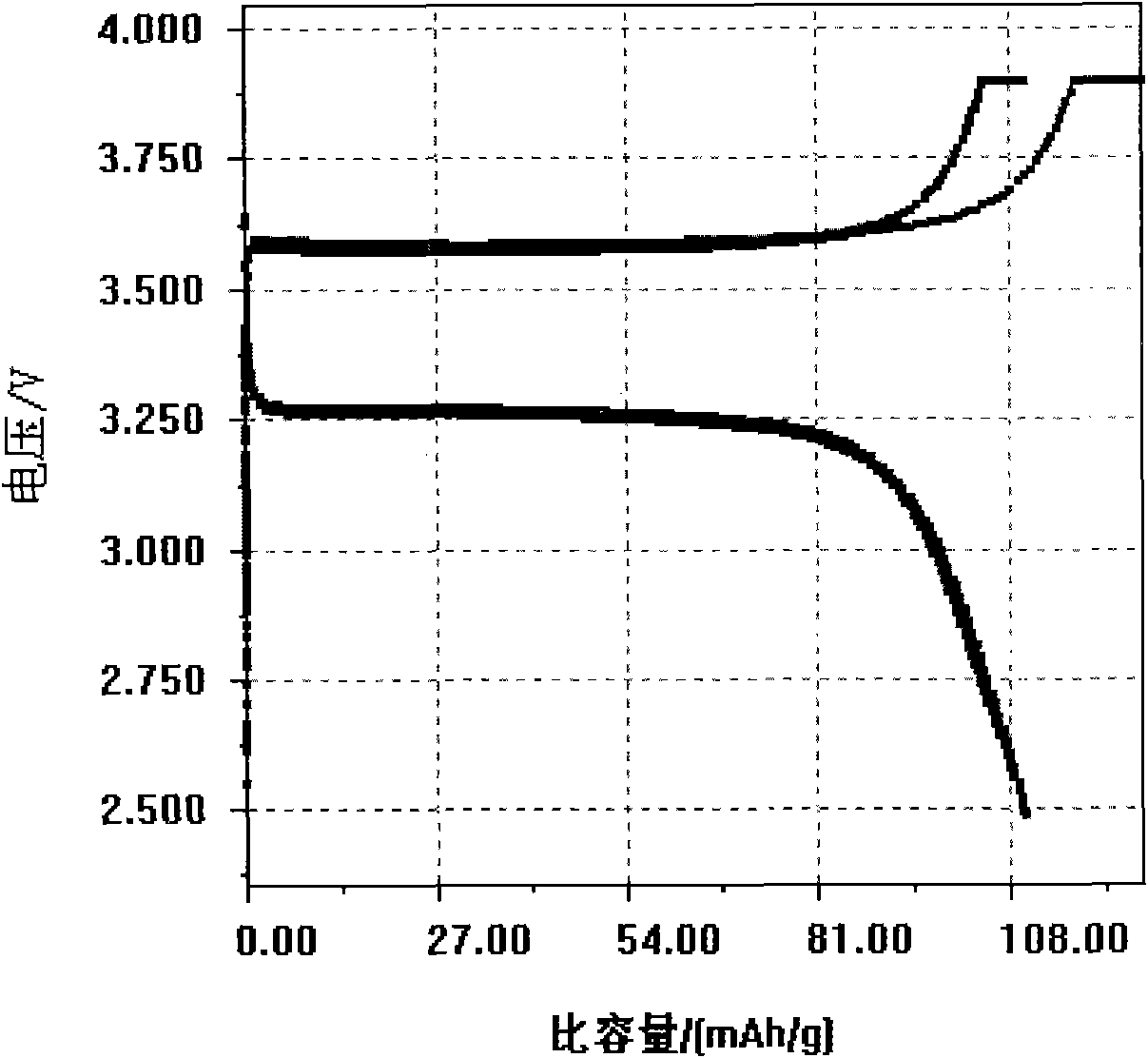
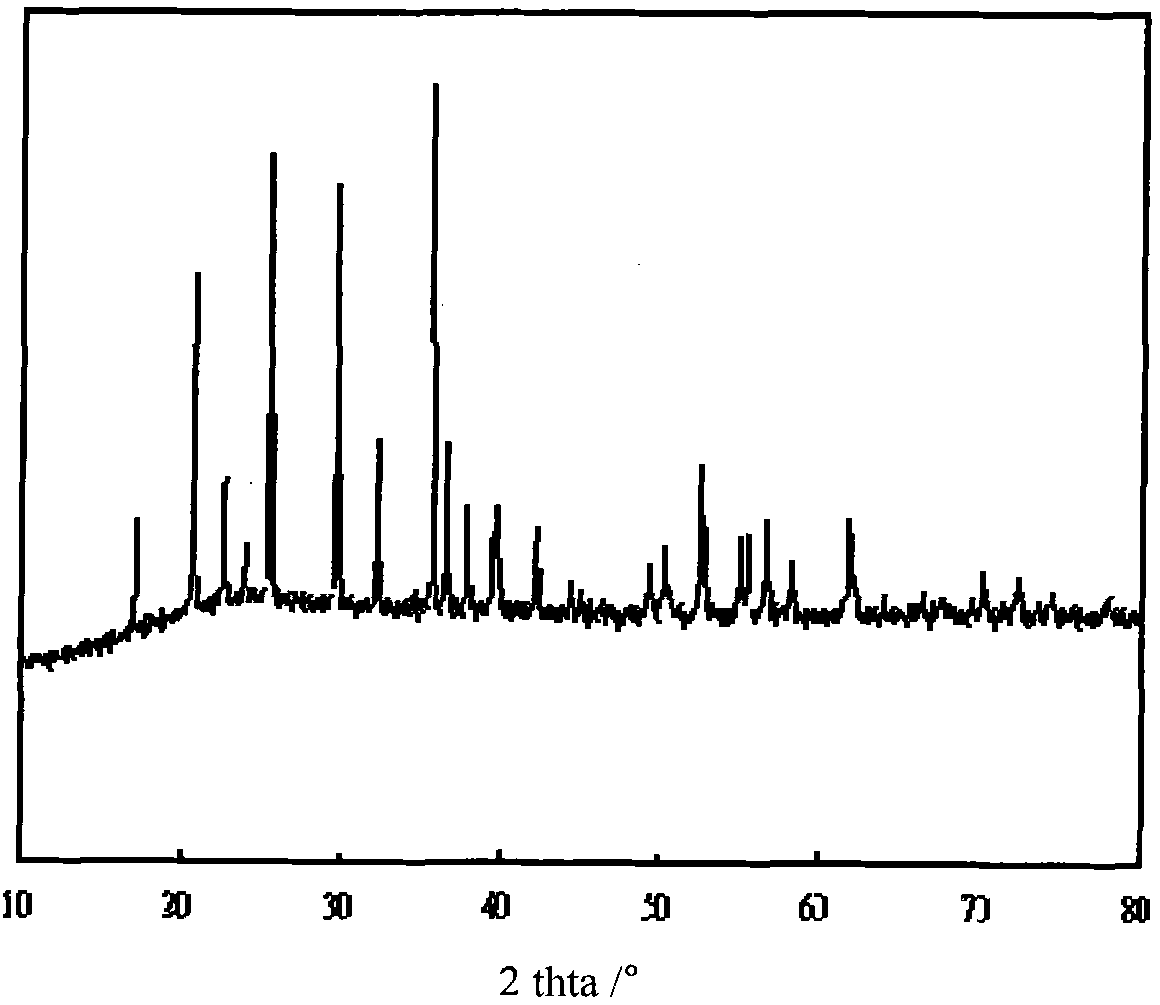
[0031] figure 1 is its X-ray diffraction pattern, and the obtained product is pure-phase olivine-type lithium iron phosphate. figure 2 It is the first and second charge and discharge curves of lithium i...
Embodiment 2
[0033] Dissolve 61 grams of ATMP in 100 grams of water, stir well until it is completely dissolved, add 80.8 grams of ferric citrate and 8.4 grams of lithium hydroxide to mix, and heat and stir in a water bath at 100°C for 2 hours, then remove the water to obtain ferric phosphate Lithium precursor. Move the precursor into a high-temperature atmosphere furnace, heat up to 400°C at a rate of 5°C / min in an atmosphere of argon, nitrogen or nitrogen-hydrogen mixture, roast at a constant temperature for 4 hours, and then raise the temperature to 600°C at the same rate, Calcined at a constant temperature for 10 hours and then cooled naturally to room temperature to obtain lithium iron phosphate, a positive electrode material for a lithium ion battery.
Embodiment 3
[0035] First dissolve 42 grams of HEDP in 100 grams of water, stir well until completely dissolved, add 49 grams of ferric nitrate, 1.8 grams of glucose and 8.4 grams of lithium hydroxide to mix, and at the same time heat and stir in a water bath at 100°C for 2 hours to remove water, namely Obtain lithium iron phosphate precursor. Move the precursor into a high-temperature atmosphere furnace, heat up to 400°C at a rate of 5°C / min in an atmosphere of argon, nitrogen or nitrogen-hydrogen mixture, roast at a constant temperature for 4 hours, and then raise the temperature to 600°C at the same rate, Calcined at a constant temperature for 10 hours and then cooled naturally to room temperature to obtain lithium iron phosphate, a positive electrode material for a lithium ion battery.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com