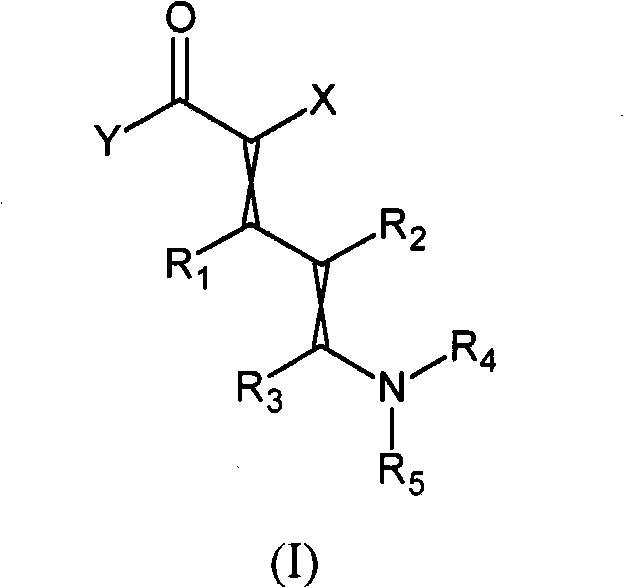
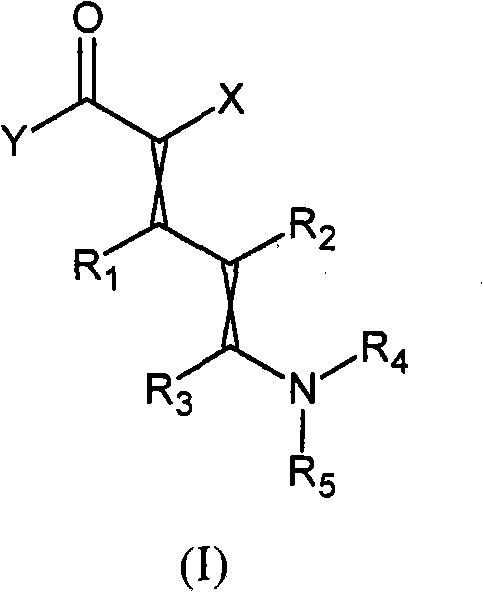
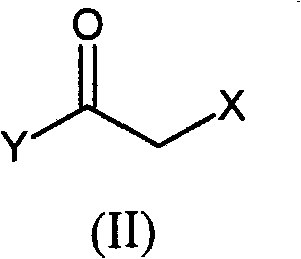
Novel precursors
A compound and general formula technology, applied in the field of synthesis of peripheral inhibitors, can solve the problems of liver damage, short half-life in vivo, side effects of central nervous system, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Embodiment 1: Preparation of ethyl 5-(dimethylamino)-2-(2,2,2-trifluoroacetyl)-penta-2,4-dienoate
[0060] equipped with a mechanical stirrer, thermometer, dropping funnel and N 2 In an inlet 5L three-neck round bottom flask, 51.60g (0.706mol) of N,N-dimethylformamide was dissolved in 2L of DCM. in N 2 The clear solution was cooled to 0° C. under 0°C, and oxalyl chloride (89.7 g, 61.7 ml, 0.707 mol) was added dropwise over 30 minutes. During the addition, a white precipitate formed. The reaction mixture was allowed to warm to room temperature and stirred for 2 hours. Cool to 0°C again and add 1-(ethyleneoxy)butane (141.20 g, 182 ml) dropwise keeping the internal temperature below 5°C. Addition continued for 45 minutes. Then remove the cooling bath and react under N 2 Stir for 2 hours. Once again the light yellow homogeneous solution was cooled to -5°C and ethyl trifluoro-acetoacetic acid (99.97 g, 0.543 mol) was added rapidly followed by triethylamine (157 g, 217...
Embodiment 2
[0062] Example 2: Preparation of ethyl 5-(dimethylamino)-2-(2,2,2-trifluoroacetyl)penta-2,4-dienoate
[0063] In a 20ml pear-shaped flask, ethyl 4,4,4-trifluoro-3-oxobutanoate (oxobutanoate) (3.00g, 16.29mmol), 3-(dimethylamino)acrolein (2.63g, 26.6 mmol) was taken up in acetic anhydride (5.8 ml) to give a brown solution. The reaction mixture was stirred for 10 minutes. Thin layer chromatography (TLC) showed the reaction was complete. The reaction mixture was dissolved in DCM. The organic phase was washed with 1N HCl solution, water, and then with MgSO 4 dry. After filtration and evaporation, the resulting dark red oil crystallized from a mixture of petroleum ether and diethyl ether.
[0064] Yield: 3.56g (83%), mp: 70°C
Embodiment 3
[0065] Example 3: Preparation of 5-(dimethylamino)-2-(2,2,2-trifluoroacetyl)penta-2,4-dienenitrile (dienenitrile)
[0066] equipped with a magnetic stirrer, thermometer, dropping funnel and N 2 In an inlet 500ml three-neck round bottom flask, 8.88g (121.6mmol) of N,N-dimethylformamide was dissolved in 350ml of DCM. in N 2 The clear solution was cooled to 0° C. and oxalyl chloride (10.6 ml, 15.43 g, 121.6 mmol) was added dropwise over 30 minutes. During the addition, a white precipitate formed. The reaction mixture was allowed to warm to room temperature and stirred for 2 hours. Cool to 0°C again and add 1-(ethyleneoxy)butane (24.32g, 31.4ml) dropwise keeping the internal temperature below 5°C. Addition continued for 30 minutes. Then, remove the cooling bath and react under N 2 Stir for 2 hours. Once again the pale yellow homogeneous solution was cooled to -5°C, and 4,4,4-trifluoro-3-oxobutyronitrile (12.82 g, 93.57 mol) was added rapidly, followed by triethylamine (30.7...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Yield | aaaaa | aaaaa |
| Yield | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com