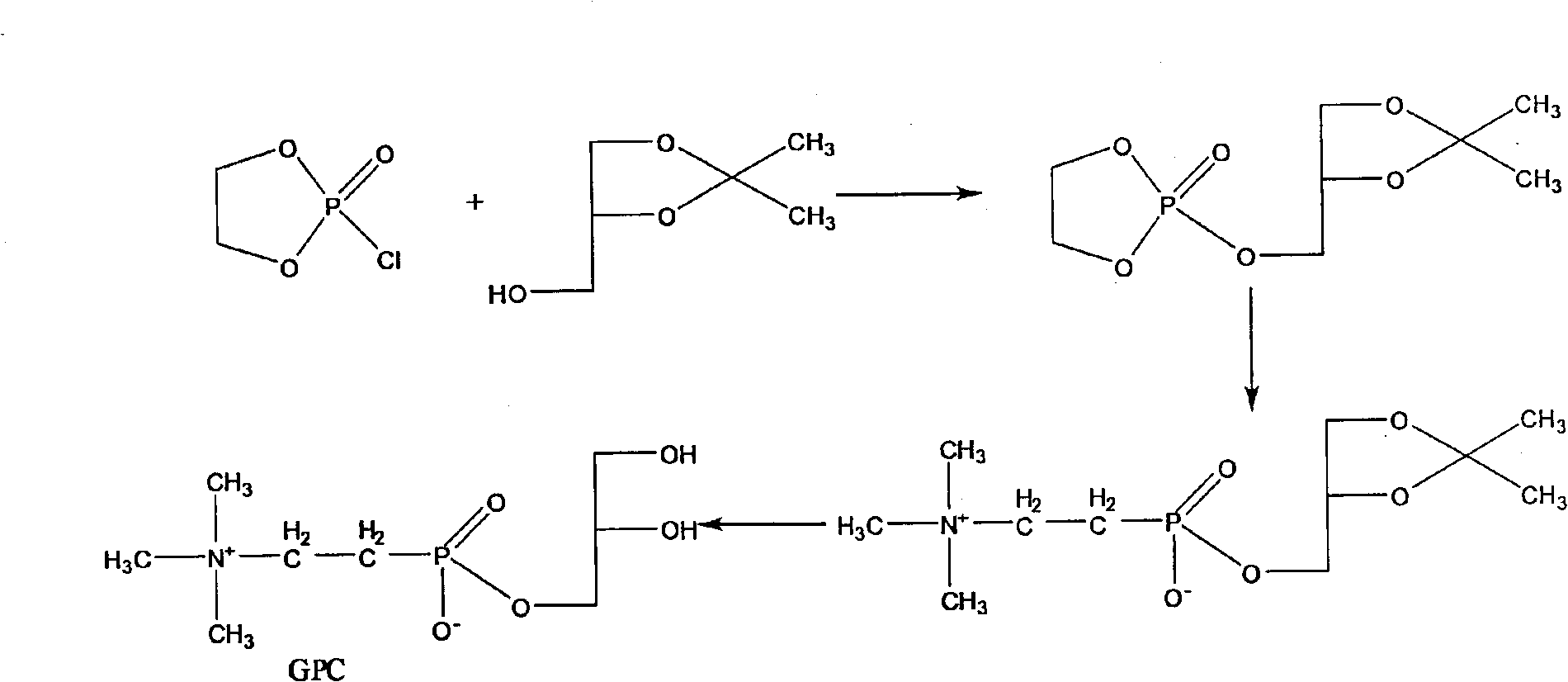
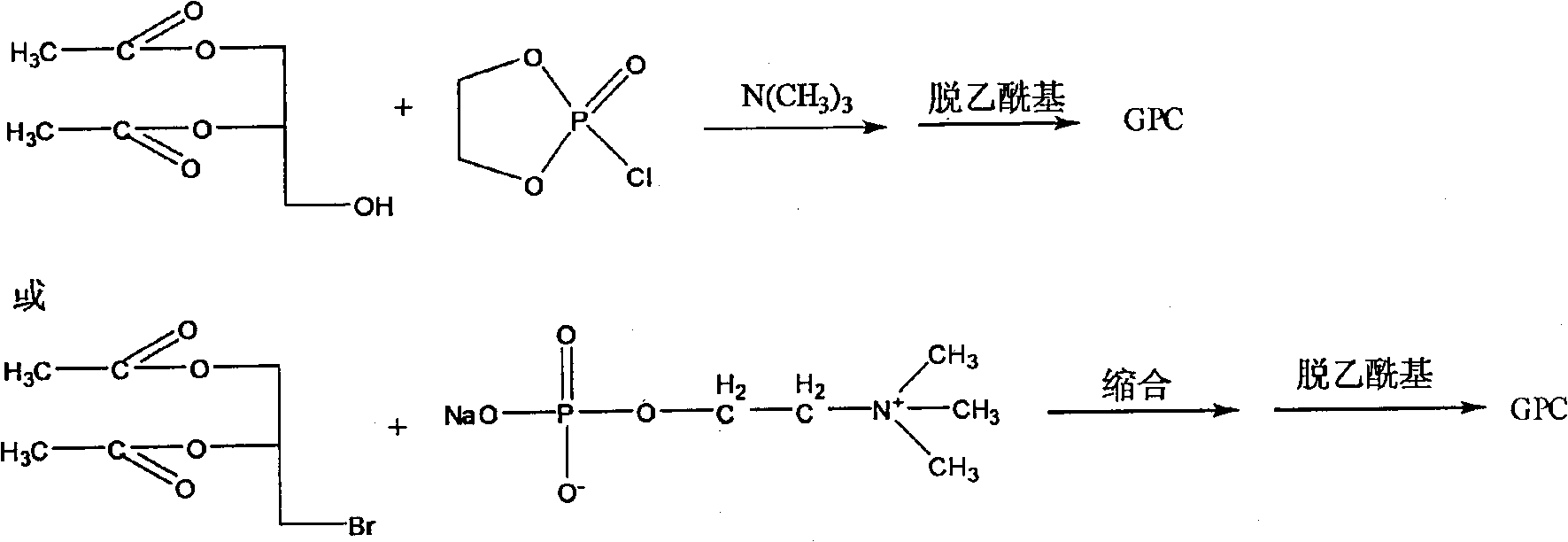
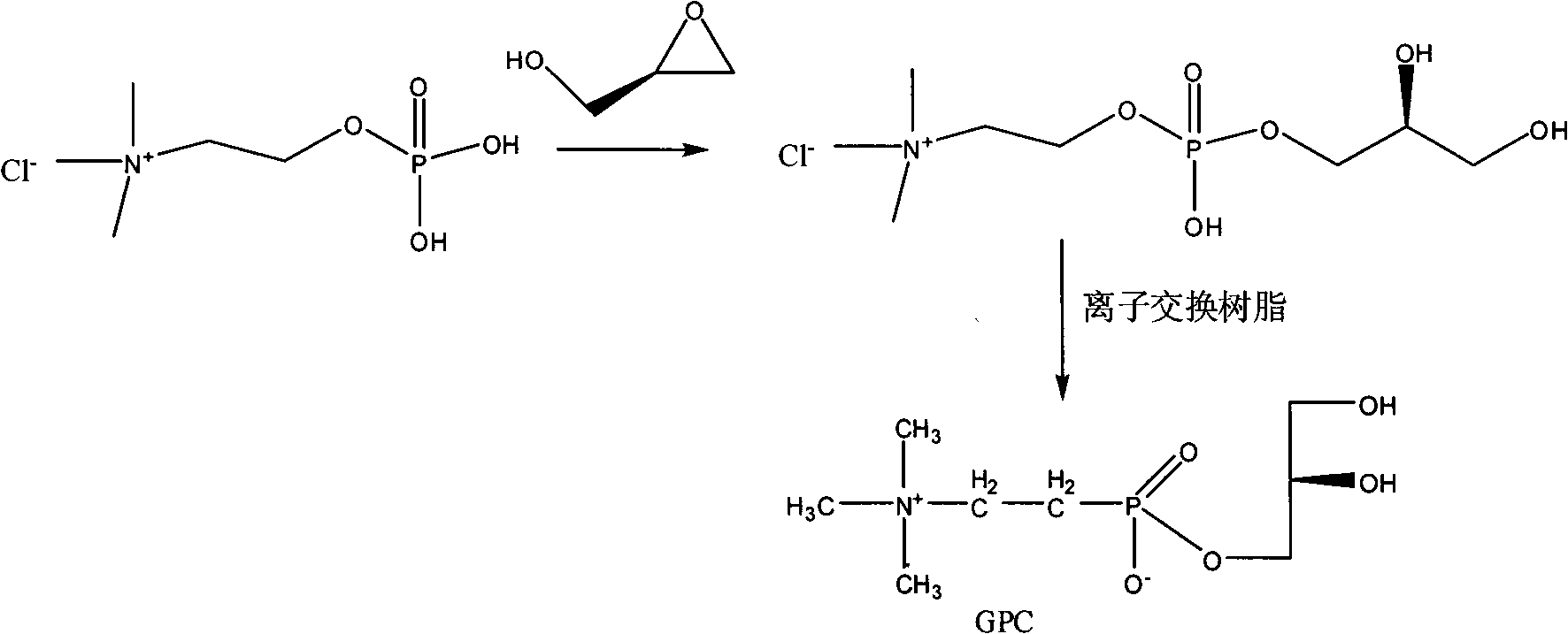
One-step method for preparing raceme DL, D or L-a-glycerin phosphorus acyl choline
A technology for glycerol phosphoryl choline and phosphoryl choline, which is applied in directions such as phosphorus organic compounds, can solve the problems of complicated process steps, unsuitable for industrialized production and the like, and achieves the effect of simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] In a 500 ml four-necked reaction flask, add 135 ml of water, start stirring and add 30 g of phosphorylcholine chloride calcium salt (containing 4 crystal water), after all dissolve, add 9.75 g of anhydrous sodium carbonate and 90 ml of water dropwise at room temperature The sodium carbonate solution made, the dropping time is controlled at 30~45 minutes, stir at room temperature for 1.0 hour after dropping, filter, the filter cake is washed with 20ml of water, the washing liquid is incorporated into the filtrate, and placed in a reaction flask under reduced pressure below Evaporate the water to dryness at 60 degrees to obtain a white solid, add 240 milliliters of absolute ethanol, stir and add 14 grams of R-3-bromo-1,2-propanediol, heat up and make it reflux until the TLC reaction is completed, ( The reaction formula of the synthesis reaction is shown above) cooling, filtering, washing the filter cake with a small amount of ethanol, combining the filtrates, distilling th...
Embodiment 2
[0028] In the same manner as in Example 1, 14 grams of R-3-bromo-1,2-propanediol was changed to 10 grams of R-3-chloro-1,2-propanediol to obtain 18.3 grams with an optical rotation of -2.7° and a purity of over 99.48%. White L-α-GPC crystals.
Embodiment 3
[0030] Change 30 grams of phosphorylcholine calcium salt into 17 grams of phosphorylcholine according to Example 1, add 2 grams of sodium hydroxide after being dissolved in water, add R-3-chlorine 1,2-propanediol dropwise after becoming a clear liquid, and Operation method (that is, the same as in Example 1, the same below) can finally obtain 18.6 grams of GPC, with an optical rotation of -2.6° and a purity of 99.38% white L-α-GPC crystal.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com