Quality analysis method for loins-strengthening and kidney-invigorating oral liquid
A kidney oral liquid, quality analysis technology, applied in the analysis of materials, material separation, pharmaceutical formulations, etc., can solve the problems of large interference, many impurities, interference of medicinal ingredients, etc., to achieve the effect of quality control, strong specificity, and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Example 1 A quality analysis method of Zhuangyao Jianshen Oral Liquid
[0020] The determination of formononetin content by HPLC includes the following steps:
[0021] a. Prepare the test solution
[0022] Take 40ml of Zhuangyaojianshen oral liquid, add petroleum ether for extraction 2 to 3 times, 60ml each time, discard the petroleum ether; add chloroform for extraction 2 to 3 times, 60ml each time, combine the extracts, evaporate to dryness, and residue Add methanol to the volume to 10ml, shake well, filter, and use as the test solution;
[0023] b. Prepare the reference solution
[0024] Take formononetin reference substance, add methanol to make 1ml solution containing 20μg formononetin to obtain the reference substance solution;
[0025] c. Determination
[0026] Chromatographic conditions: octadecylsilane-bonded silica gel is used as filler, and acetonitrile-1% formic acid solution with volume ratio of 38~42:58~62 is used as mobile phase; detection wavelength is 290nm; the n...
Embodiment 2
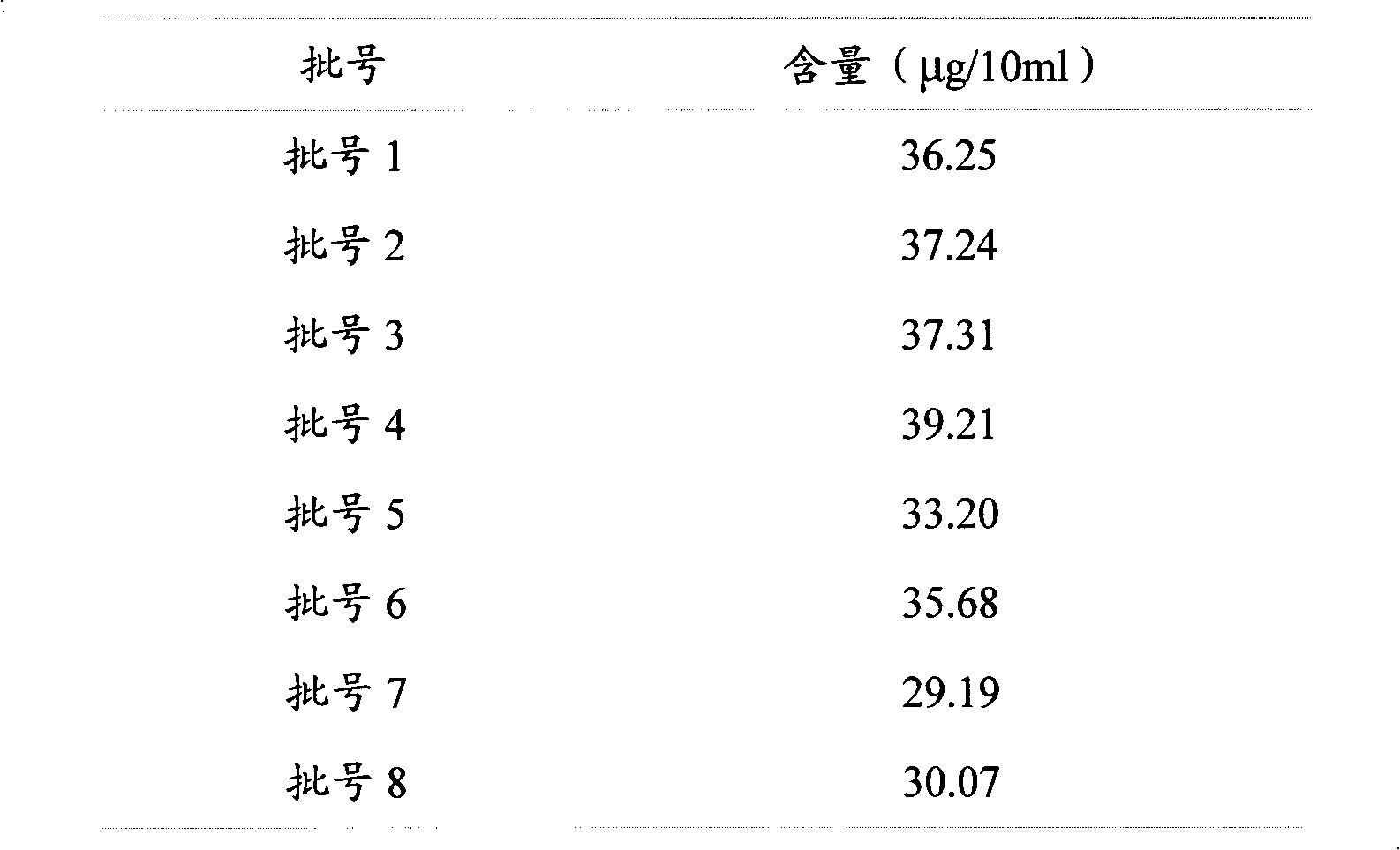
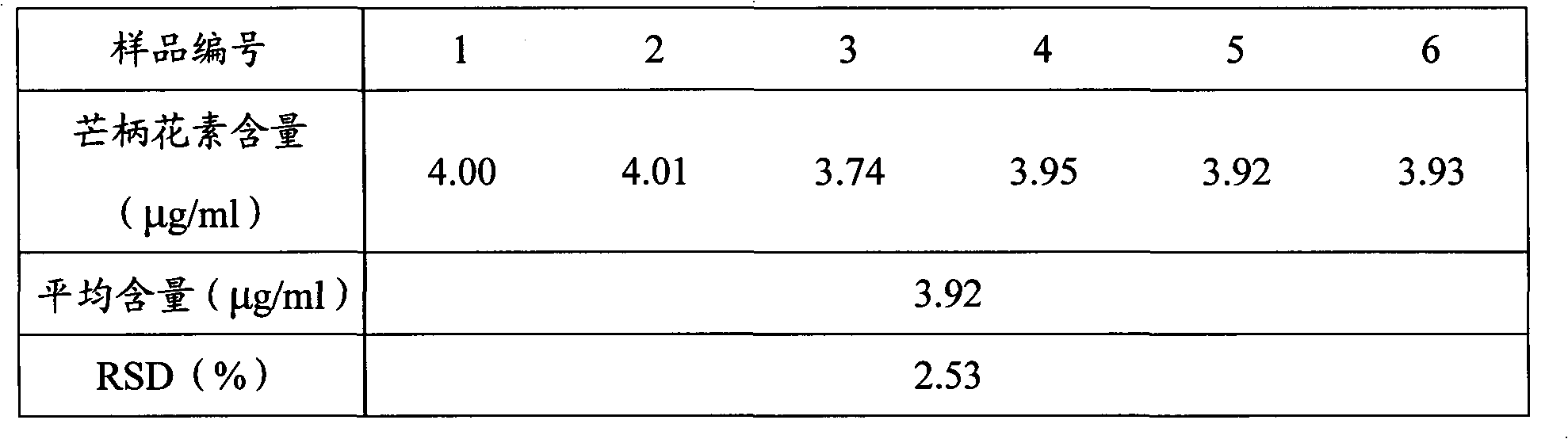
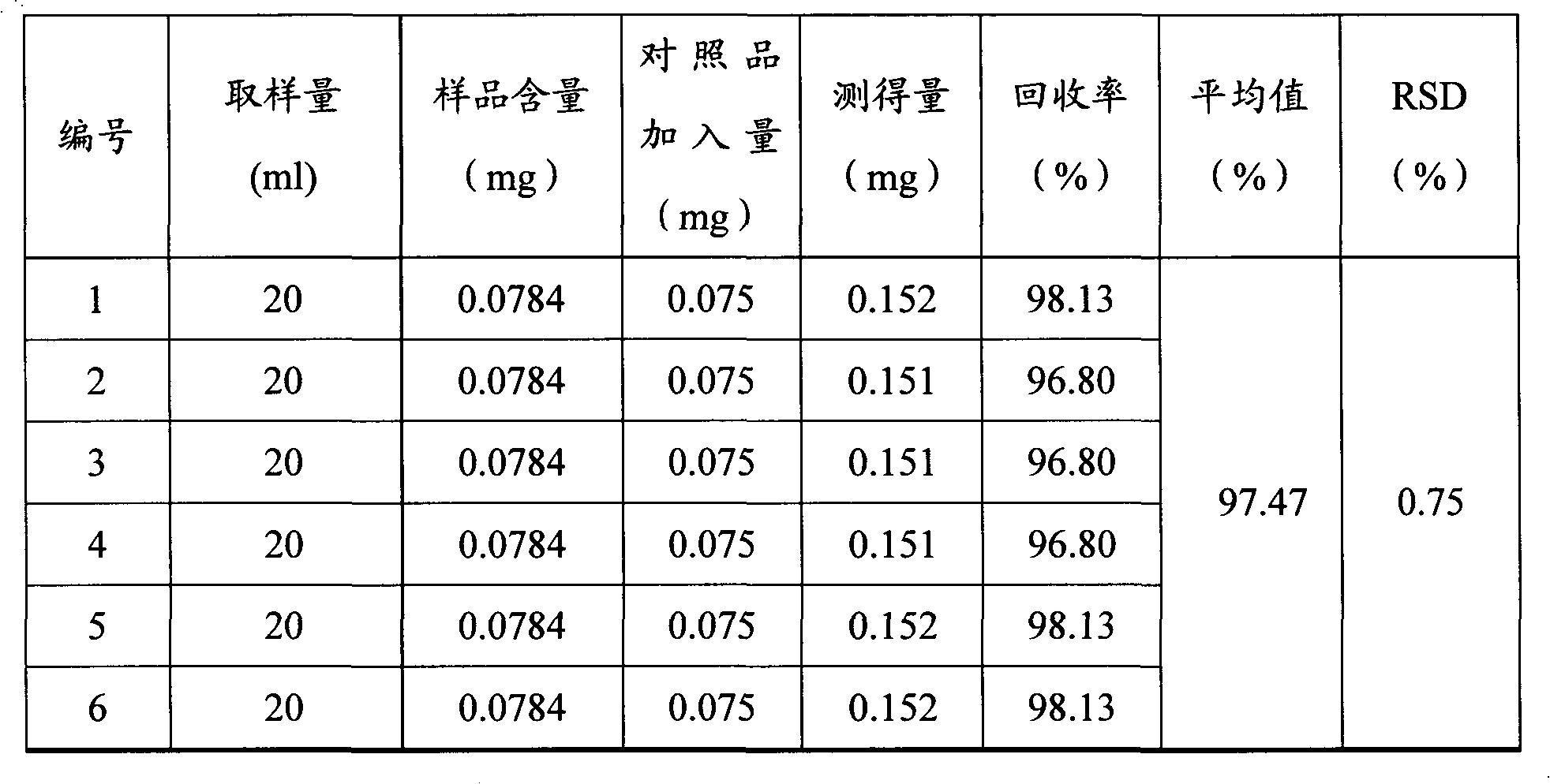
[0031] Example 2 Conditional screening and methodological verification of a quality analysis method for Zhuangyao Jianshen Oral Liquid
[0032] 1. HPLC chromatographic conditions screening
[0033] The existing literature reports that there is absorption at 249nm and 254nm. The spectrum measured by the diode array detector shows that there is absorption at 249nm, 254nm, 290nm, 327nm, and the maximum absorption is 290nm. The sensitivity is high at this wavelength and the peak shape is sharp. , The resolution is greater than 1.5, and the peak time is within 10 minutes, which saves the detection time. Therefore, the chromatographic conditions are determined as follows: octadecyl silane bonded silica gel is used as a filler, and acetonitrile at 38~42:58~62 is -1% The formic acid solution is the mobile phase; the detection wavelength is 290nm. The number of theoretical plates should not be less than 4000 based on formononetin peak.
[0034] 2. Preparation of the test article
[0035] Ref...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com